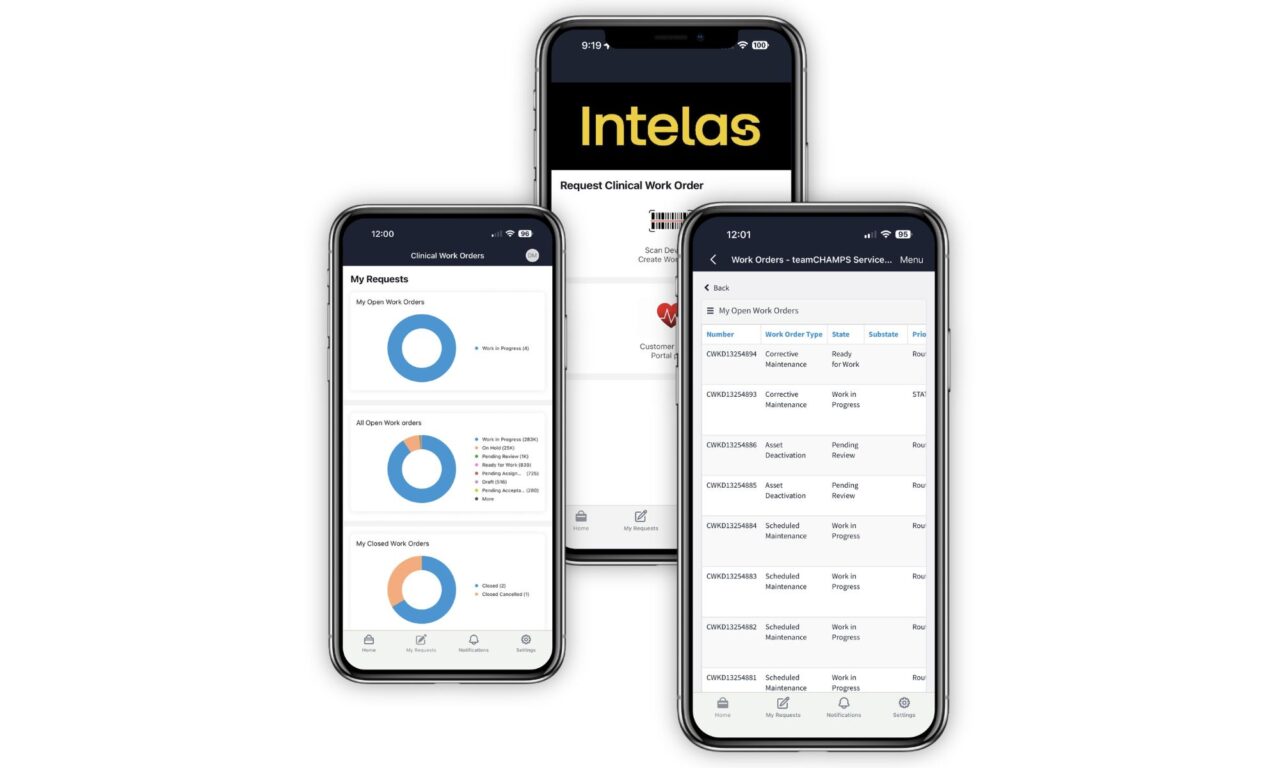
Intelas Introduces Mobile App for Faster, Trackable HTM Service Requests
The new mobile experience connects technicians, clinical staff, and hospital leaders on a single platform to help accelerate issue resolution and improve visibility across medical equipment service.