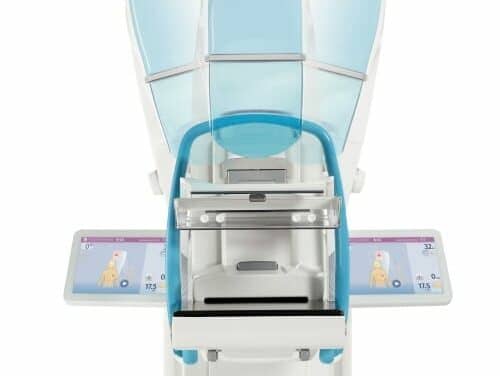
First U.S. Facility Installs Mammomat B.brilliant
GRACE Breast Imaging & Medical Spa is the first U.S. facility to install Siemens Healthineers’ Mammomat B.brilliant, a mammography system for breast cancer detection, patient comfort, and workflow efficiency.