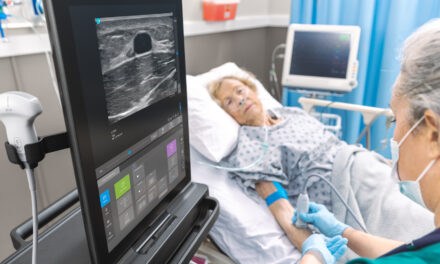
Signostics has received FDA 510(k) clearance for Uscan, a mobile-connected ultrasound visualization device for urology applications. Uscan, which actively recognizes the 3D contours of the bladder, acquires up to 256 bladder slices—32 times more than conventional bladder scanners. It also provides real-time ultrasound imaging of the kidneys, pelvic floor, prostate, gallbladder, bladder stones, and catheter emplacement.
Uscan also offers integrated middleware and can be used in emergency department, maternity, pediatrics, oncology, rehabilitation, aged care, and home nursing settings. The system’s removable probe, touchscreen tablet, and handheld displays further suit it for on-the-go clinical care.
Kevin Goodwin, CEO of Signostics, says these features make Uscan a major asset in the global health care field. “Uscan doesn’t just scan, it sees—providing intelligent urologic visualization by leveraging science from current-day computer vision algorithms aimed at more efficient and confident point-of-care clinical decision-making,” he says. “[It] will exceed historical industry standards for bladder volume measurement accuracy.”
The device is also compatible with Android operating systems and has built-in Wi-Fi and Bluetooth connectivity that enables interoperability with electronic health record systems. In addition, Uscan, which comes with a 5-year product warranty and requires no annual calibration, eliminates the need for extensive training. The product will be commercially available in the United States next month.
For more information about this device, visit Signostics.