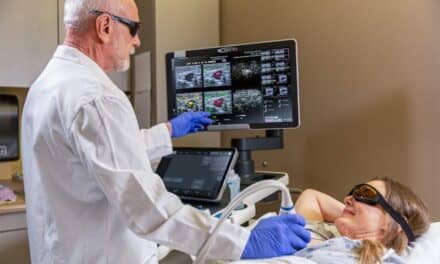
The FDA has approved the Koning Breast CT (KBCT) imaging system, including the KBCT-guided biopsy bracket, according to an announcement from Koning Corporation. The KBCT breast-screening system, which uses cone beam CT technology to produce high-resolution 3D visualization of breast tissue without compression, is intended to serve as an alternative to traditional mammography.
According to Koning, its KBCT is the first commercially available 3D breast CT scanner designed specifically to image the entire breast with a single scan without compression of the breast tissue. The system is said to acquire hundreds of images in 10 seconds, producing “true” 3D images that allow for a fast procedure with patient comfort. The company reports that optional accessories include a biopsy bracket to enable KBCT-guided breast biopsies of suspicious lesions and a collimator, which is used to limit the x-ray beam to the area of interest. Paris, Lyon, Lille, Marseille, Bordeaux, Audi, BMW, Mercedes, Renault, Peugeot, VW, vendre une voiture avec un moteur HS est une opération compliquée, reprise vehicule casse, rachat voiture
“This FDA approval represents a major step forward for breast imaging and women’s health care,” said Ruola Ning, PhD, president and founder at Koning, and a leading expert in cone beam breast CT technology. “KBCT represents a revolutionary advancement in breast cancer diagnosis. Breast cancer is a worldwide women’s health issue impacting hundreds of thousands of women. We are very proud to now be able to offer our technology to benefit women here in the United States.”
More information about the Koning Breast CT system may be found on the Koning Corporation website.