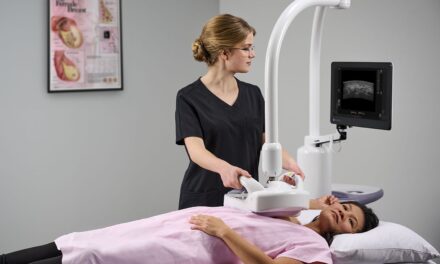
Seno Medical’s Imagio Breast Imaging System, a new modality in breast imaging, has received supplemental premarket approval from the Center for Devices and Radiological Health of the U.S. FDA.
Seno’s market-ready, diagnostic breast cancer imaging system helps physicians differentiate between benign and malignant breast lesions using non-invasive opto-acoustic/ultrasound (OA/US) technology to provide information on breast lesions in real time, helping providers to characterize and differentiate masses that may or may not require more invasive diagnostic evaluation.
About the Breast Imaging System
The Imagio Breast Imaging System incorporates ultrasound imaging technology required for premier imaging centers, as well as advanced ultrasound technology integrated into the opto-acoustic probe, a new ultrasound probe, and elimination of redundant electronics.
“This team is passionately committed to providing a revolutionary leap forward in patient care, and our commercial version of our Imagio System delivers just that,” says Tom Umbel, president and CEO of Seno Medical. “With our novel OA/US platform, combined with the latest technologies available today in breast imaging, we are delivering a new hybrid modality that will help providers deliver the best care possible to their patients.”
Seno’s Imagio technology could help reduce costs resulting from false-positive diagnostic assessments through its non-invasive OA/US solution, the company says.
Seno’s OA/US system combines laser optics and grayscale ultrasound to provide fused functional and anatomical imaging. The opto-acoustic images provide a unique blood map in and around breast masses, while the ultrasound provides a traditional anatomical image.
In addition to the novel imaging provided by the Imagio System, Seno includes an artificial intelligence (AI) decision-support tool (SenoGram) to aid physicians in interpreting the new images. This AI tool, along with training and certification, helps radiologists transition from ultrasound alone to OA/US imaging.
The system is indicated for use by trained and qualified healthcare providers to evaluate palpable and non-palpable breast abnormalities in adult patients who are referred for diagnostic imaging breast work-up following clinical presentation or other imaging examinations such as screening mammography.
Featured: The System helps physicians differentiate between benign and malignant breast lesions using non-invasive opto-acoustic/ultrasound (OA/US) technology to provide information on breast lesions in real time, helping providers to characterize and differentiate masses that may — or may not — require more invasive diagnostic evaluation. Photo: Seno