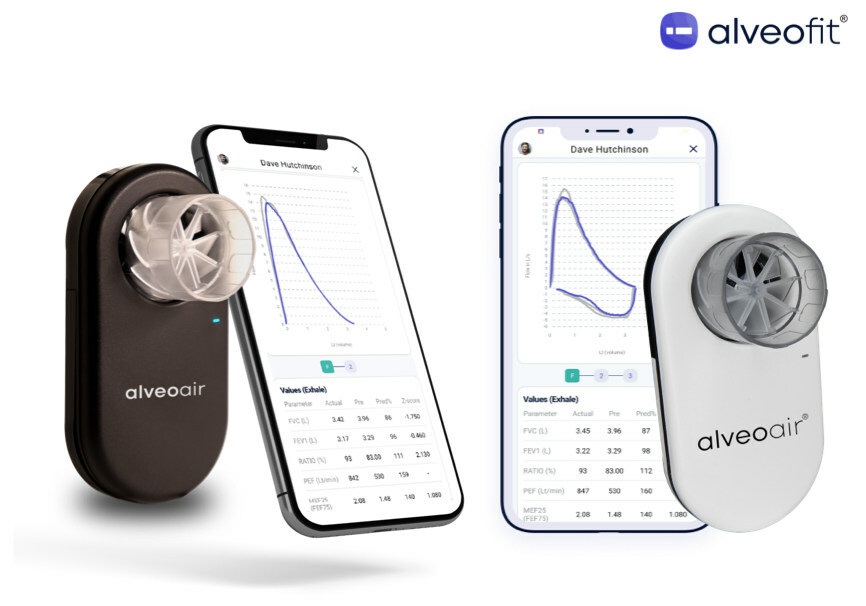
Alveofit announces that its product, Alveoair spirometer, has earned FDA clearance for distribution in the United States. FDA approval, coupled with strategic partnerships like the one with AstraZeneca, positions Alveofit for broader international expansion, particularly in the U.S. and emerging economies, according to the company.
Dr Anil Kukreja, vice president of medical affairs and regulatory at AstraZeneca Pharma India, praised the FDA clearance as a milestone, highlighting Alveofit’s role in optimized diagnosis and management of lung disorders such as asthma and COPD.
Since achieving CDSCO certification in India in June 2022, Alveoair has been adopted by over 400 healthcare facilities across India. The company has partnered with organizations like India Sweden Innovation Centre (ISIHC), NASSCOM COE, PATH, Forge Innovation & Ventures, and DST.
“It is good to see the product mature over the last couple of years and gain acceptance among the providers. I congratulate and wish Alveofit a global success with recent approval from FDA, making a difference in the lives of people in India and world over,” said Sanjeev Malhotra, CEO, NASSCOM COE.
Alveofit collaborates with prominent academic institutions such as AIIMS Delhi for clinical research and has formed an alliance with Tatvacare to extend digital respiratory care solutions to patients’ homes.