Infusion devices are capable of delivering medication such as insulin or hormones, antibiotics, chemotherapy or pain relief drugs, and nutritional solutions where other methods would be impractical or unreliable at rates from 0.1 milliliters per hour (mL/hr). It is estimated that there are millions of infusion devices in use around the world, primarily in hospitals, and that every year, more than 80% of hospitalized patients receive some form of IV therapy, reinforcing the paramount role infusion pumps play as essential tools for providing preoperative care, critical care, and pain management.
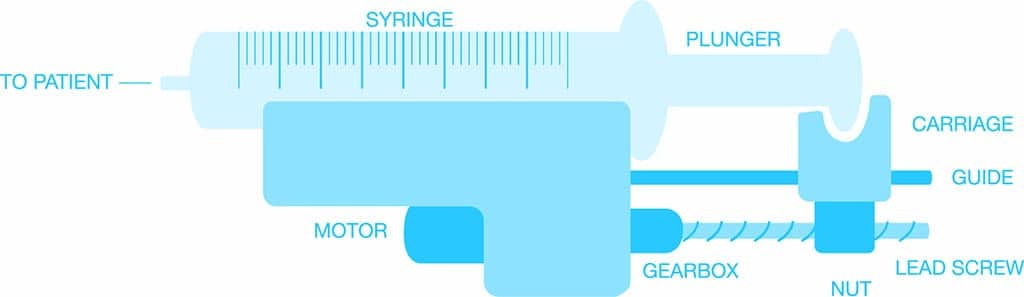
There are several different types of infusion devices commonly in use, employing a variety of mechanisms to control the flow and volume being infused for a range of purposes and environments. The most common are elementary gravity controllers, which use a clamping action to vary the flow; volumetric pumps, which employ a linear peristaltic pumping mechanism; and syringe pumps, which work by pushing a plunger to drive a syringe at a predetermined rate.
The pattern of fluid delivery is dependent on the type of pump used and typical flow patterns for volumetric, syringe, and ambulatory pumps at a flow rate of 1 mL/hr. Each pump can deliver accurate average flow rates (within manufacturer specifications) over long periods of infusion. However, syringe pumps are typically used to deliver fluid accurately over shorter periods of time.
While the vast majority work safely, infusion pumps have been involved in a number of safety incidents. In the UK alone, at least 1,000 incidents have been investigated by the Medicines and Healthcare Products Regulatory Agency (MHRA) between 2005 and 2010. The majority of these relate to overinfusion of drugs due primarily to user error involving dosage or patient data. Some incidents have been caused by product design and engineering or software malfunction.1,2 Therefore, it is important that those with responsibility for medical device safety and performance arrange for IV devices to be regularly tested and evaluated to ensure that they are functioning to the manufacturer’s specification and within clinical and environmental expectations.
Flow Measuring Principles
The primary aim of testing is to verify that the device delivers the required flow rate, volume, and bolus accurately; that occlusion alarms are activated when necessary; and that the device is safe for patient and operator use. It’s important that testing conditions mirror real-life settings and that they reflect what the manufacturer recommends to ensure that the equipment is working within its specification.
Testing can involve a variety of methods, but the essential criterion is to measure the accuracy of the delivered volume and flow rate over a range of time periods (typically between 10 minutes and 1 hour, in tests conducted over several days). Common flow measuring principles are as follows:
• Volumetric. Flow is calculated after a certain volume has been delivered. The greater the volume over a certain time, the greater the flow.
• Mass. Flow is calculated based on the temperature difference between two points within the sensor. The greater the temperature difference, the lower the flow.
• Bubble tracking. Flow is calculated based on the displacement of an inserted air bubble into the flow sensor part. The greater the displacement, the greater the flow.
• Pressure-based. Flow is regulated within the flow sensor to a set line pressure. The greater the potential pressure that is built up in the line, the greater the flow rate.
• Displacement of syringe plunger. Flow rate is calculated based on volume displaced by the syringe plunger over time. The syringe type and volume are required to provide an accurate calculation.
Occlusion alarm pressures as well as bolus delivery have to be tested to maintain the performance of the infusion device, especially in PCA devices where the bolus is self-medicated. A visual inspection and electrical safety test should also be considered to make sure all aspects of patient safety and instrument reliability are covered during the test procedure.
Testing Methods
Optimal infusion is the ability of a device to reliably deliver the prescribed dosage and volume to the patient, at a pressure that overcomes all baseline and intermittent resistance, while causing no harm to the patient.3 The reliability of infusion pumps is extremely important because these devices are used for patients who could be in a critical condition. Moreover, the safety incidents associated with infusion devices demonstrate that there is a need to adequately validate the accuracy and performance of these devices.4
There are several methods for testing reliability. Users are increasingly moving away from basic measurements such as weighing scales and burettes, where continuous user input is required to ensure accuracy, toward automatic flow analyzers, which record real-time results graphically and comply with both the IEC 60601-2- 24 standard and manufacturer’s specifications, as outlined in service manuals. Furthermore, it is important to ensure that the accuracy of an infusion system is looked at in its entirety, taking into account all possible inaccuracies. For example, the syringe used and other external equipment, including the tubing set, could increase the inaccuracy to 10%. It is therefore generally accepted that the testing method and equipment have to be more accurate than the equipment under test.2
Common methods for measuring volume or flow rate are as follows:
- Direct volumetric, using either graduated cylinders or burettes.
- Derived mass measurement, using a measurement vessel and weighing scales.
- Vernier calipers and dial gauges, to provide a direct reading of the distance measured with high accuracy and precision.
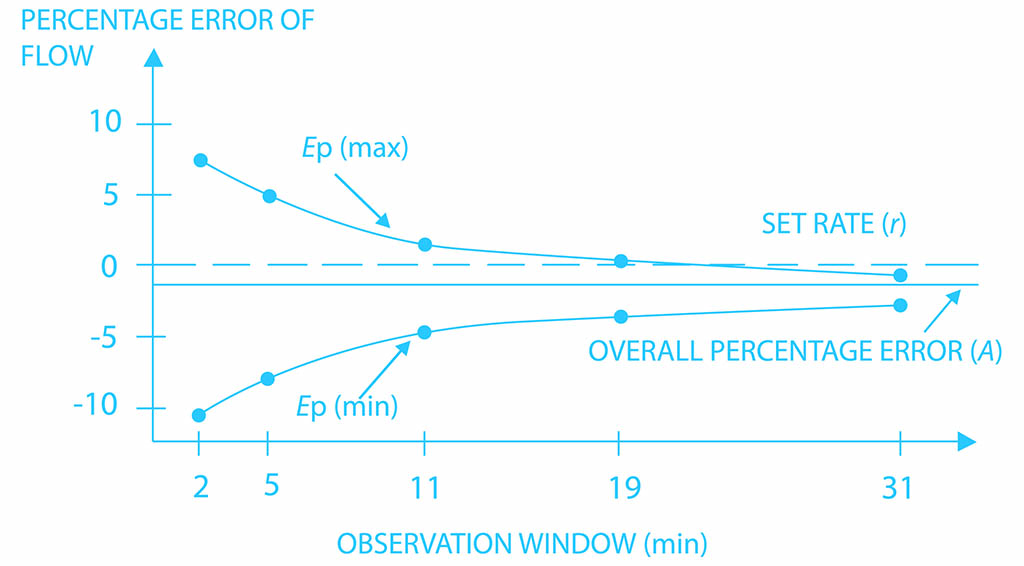
Trumpet curves show the accuracy performance of an infusion device at set intervals in the second hour of infusion and during the final hour of infusion, as required by IEC 60601-2-24. The migration toward automatic solutions has resulted in growing interest in testing equipment that uses a pressure-based system to provide instantaneous flow readings and to quickly verify the infusion devices’ accuracy and performance to a resolution of 10 µL/hr.
Reliability and Accuracy
Whether it is with a burette (direct volumetric measurement), weighing scales (derived mass measurement), or through the use of automatic analyzers, the most important consideration when selecting a method of testing is that it provides reliable and accurate results.
Direct volume measurements are techniques that provide a good degree of accuracy without the need to worry about the design or performance accuracy of another piece of equipment. However, they are labor intensive and require continuous user input. Automated electronic devices combine the various manual test methods with the ability to conduct infusion device performance accuracy analysis with little user input, as well as the ability to test multiple devices simultaneously.
The volume of fluid delivered is used to measure the system’s volumetric accuracy. Other tests determine the performance accuracy of the device to maintain safety for staff and patients who will use the equipment. Most manufacturers specify system accuracy under stated test conditions, including the giving set, temperature of test, rates, and so forth. This indicates that there are a number of elements that can affect the accuracy. Therefore, the device being tested must be used within the recommended specification to maintain the given limits of accuracy.
Most adverse incidents are eventually identified as user error. Good design can contribute to minimizing such user error. Work has been initiated to develop a formal ergonomic testing procedure for application to all devices. At present, user instructions are assessed for clarity and readability, conciseness, and indexing. Procedures for using the device are systematically worked through on the bench after other testing is completed. Any hazardous potential misuse is noted. For all available alarms, the reliability, readability of text displayed, alarm tone quality, positioning of alarm lights, and methods of silencing alarms are tested as part of the ergonomic assessment of the device.5
The technological advances in infusion pumps over the past 40 years have transformed the treatment of patients in hospitals, and have afforded the ability to infuse fluids on an outpatient basis or in a home environment, enabling patients to receive treatment while going about their daily lives.6 Now, the advancing technology of new infusion pumps means they can infuse very low volumes and for extended periods. Therefore, measuring performance accuracy needs to keep up with the technology of new pumps to be able to satisfactorily evaluate infusion pumps in terms of volume and flow rate, occlusion, and bolus measurements.
Katherine Summers, MEng, is product specialist for Rigel Medical, Peterlee, UK. For more information, contact 24×7 editorial director John Bethune at [email protected].
References
1. MHRA. (2010). Infusion systems device bulletin. Safeguarding public health.
2. Davis W. (2010). Infusion device. Available at: http://www.ebme.co.uk/arts/infusion/inf_train.php. Accessed August 27, 2013.
3. Paul Scott. (2013). What is a dial gauge? Available at: http://www.wisegeek.com/what-is-a-dial-gauge.htm. Accessed August 25, 2013.
4. Zhang P, Wang SY, Yu CY, Zhang MY. Design of occlusion pressure testing system for infusion pump. J Biomed Sci Eng. 2009;2(6):431-434.
5. Bath Institute of Medical Engineering. (2005). Test methods and protocols. Available at: http://www.bath.ac.uk/bime/evalcentre/publications/protocols.pdf. Accessed August 28, 2013.
6. Ostendarp D. (2010). A brief history of infusion pumps. Available at: http://www.articleonlinedirectory.com/322515/a-brief-history-of-infusion-pumps.html. Accessed August 25, 2013.





Good overview of infusion pump testing but I can’t follow the logic of “… arrange for IV devices to be regularly tested and evaluated to ensure that they are functioning to the manufacturer’s specifications…” if “[t]he majority of these [incidents] … due primarily to user error… caused by product design and engineering or software malfunction.” Seems to be a non sequitur that serves well a manufacturer of IV device testers.
Hello,
I am in searching for software that will do real-time flow accuracy testing and provide output in the percentage of error flow, Please let me know if someone has used validated software that is in the market for drug products.
Thank you,
hello> it will be very helpful if you share the steps in number wise how syringe pump is operated……
thanks alot for your struggle