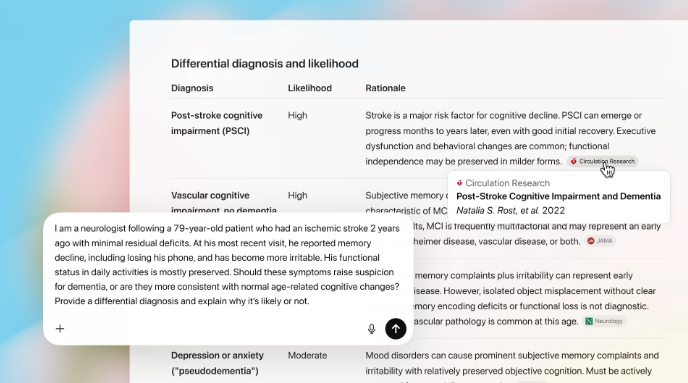
HTM’s Next Frontier: AI, Intelligent Operations, and Tech-Driven Resilience in 2026
AI is starting to touch maintenance, documentation, and device risk management in practical ways. Here’s where HTM leaders are likely to see the biggest impact in 2026.