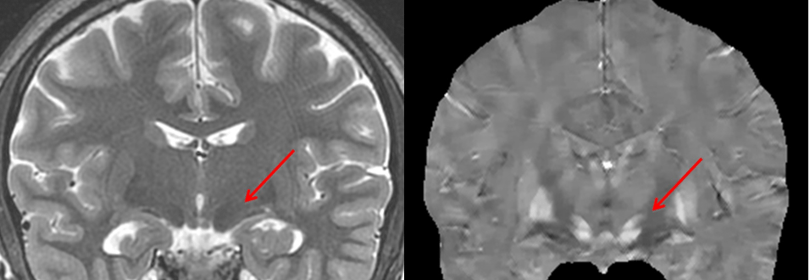
Imaging Biometrics Extends GE HealthCare Distribution Deal to Include Additional Neuroimaging Software
The deal introduces faster FTB mapping and quantitative susceptibility analysis to complement existing perfusion and contrast-enhancement solutions.