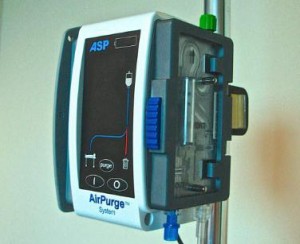
The system monitors the IV line attached to the device for any air bubbles, which, when found, are diverted to a built-in reservoir and disposed of in a collection bag. The system does not require any manual intervention.
According to the company, the AirPurge System can be integrated into an IV set up routine by installing it on the IV pole just below a fluid or blood warmer to capture smaller air bubbles that are typically generated during the infusate heating process.




