Editor’s Note: This article, written in 2015, continues to be the most-read on 24×7‘s site. We hope it can provide you with the information you need as you test SpO2 sensors.
Monitoring SpO2, the saturation percentage of oxygen in the blood, has become a standard of patient care across the globe. Almost every patient monitor has a built-in or attachable capability to monitor this crucial vital sign. SPO2 is an indirect and noninvasive method of measuring oxygen saturation in blood. It should be tested along with all the other physiological parameters during preventative or corrective maintenance on a patient monitor, or stand-alone device.
The Technology
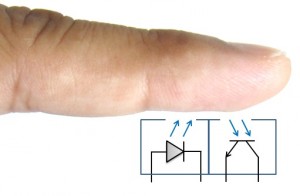
SpO2 is measured at the periphery, usually a finger, and is one measure of the health of the cardiovascular and respiration systems. A pulse oximeter noninvasively measures the oxygen saturation of a patient’s blood. This device consists of a red and an infrared light source, photo detectors, and a probe to transmit light through a translucent, pulsating arterial bed, typically a fingertip or earlobe. Oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) absorb red and infrared light differently. The percentage of saturation of hemoglobin in arterial blood can be calculated by measuring light absorption changes caused by arterial blood flow pulsations.
A variety of factors can affect the accuracy of SPO2 measurement, including skin conditions, pigment, wounds, scar tissue, tattoos, nail polish, hypothermia, anemia, medication, light interference, and movement.
SPO2 is measured using a sensor, usually attached to the patient’s finger. There are two methods of SpO2 technology: transmissive and reflective. The transmissive method is the more commonly used of the two. As shown in figure 1, transmissive technology transmits red and infrared light through the finger to a photo detector.
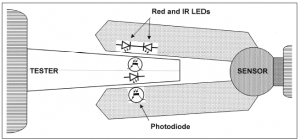
Figure 1: Transmissive technology, the most commonly used form of clinical pulse oximetry. Click to enlarge.
The other method used for SPO2 relies on reflective technology. As shown in Figure 2, this method has the transmitter and receiver in the same plane. Reflective SPO2 sensors can be placed on other areas of the anatomy than the finger, such as the forehead.
Testing
Each pulse oximetry device manufacturer must determine the accuracy of their device by conducting human testing. As Dennis J. McMahon explains in his white paper, “There’s No Such Thing as an SpO2 Simulator,”1 in a “controlled desaturation study, volunteer subjects breathe a sequence of gas mixtures of decreasing oxygen content while connected to a prototype monitor.” Arterial blood samples are then taken from the subjects to measure oxygen saturation in a clinical laboratory.
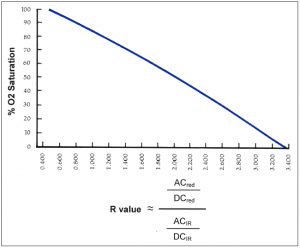
The result of this testing is a graph for that particular model of SPO2 sensor and monitor. This graph is referred to as an R-curve. As shown in Figure 3, an R-curve describes the relationship between the particular ratio of red and infrared light versus the observed oxygen saturation as collected during human testing. The R-curve is then used in the firmware for a particular instrument and for SPO2 testers.
Simulators, calibrators, and functional testers for pulse oximeters are defined in the ISO standard 80601-2-61. Unlike other types of medical devices, pulse oximeters are not designed to be calibrated outside of the factory. There are no accepted methods for verifying the correct calibration of a pulse oximeter other than human testing. Most SpO2 test equipment on the market is in the functional tester category.
According to Tobey Clarke in his book Medical Equipment Quality Assurance, patient monitors should be functionally tested at least annually.2 Most functional testers test the SPO2 sensor optically. This allows for the test of the sensor, cable, and monitor. Some functional testers input a signal directly to the monitor, only testing the monitor. Others can test the cable for continuity. Most functional testers only test transmissive technologies, not reflective.
A typical workflow for testing a patient monitor or stand-alone SPO2 monitor includes checking the physical condition, performing electrical safety tests, performing any manufacturer-recommended preventative maintenance, conducting performance testing (including alarms and other specific tests), and, finally, documenting the testing results.
Andrew Clay is a product marketing manager for Fluke Biomedical, Everett, Wash. This article is adapted from a Fluke Biomedical whitepaper. For more information, contact 24×7 editorial director John Bethune at [email protected].
References
1. McMahon DJ. There’s no such thing as an SPO2 simulator. Everett, Wash: Fluke Biomedical; 2013. Available at http://www.flukebiomedical.com/Biomedical/usen/Events/Promos/sp02-whitepaper-SOC. Accessed January 15, 2015.
2. Clark JT, Lane M, Rafuse L. Medical Equipment Quality Assurance: Inspection Program Development and Procedures. Everett, Wash: Fluke Biomedical; 2008:123.
Top photo: courtesy © Juanrvelasco | Dreamstime.com
But what makes the big difference between standard slots and video poker is the player’s decision-making: the player must decide, after each first draw, which card(s) to hold to make his final hand, the one that determines the strength of his hand and thus his potential winnings in SGD dollar casinos by onlinecasino65.sg. The obvious answer to the question “why play video poker” is that you are master of your own destiny and therefore of your winnings, unlike a slot machine where you just have to make a single click and then take a chance.





1. We agree with Andrew Clay that pulse oximeters should be tested.
2. Patient factors affect accuracy. Clinicians are aware of this but expect the pulse oximeter to work as the manufacturer claims.
3. Andrew points out that sensors emit red and infra red but does not point out that variations in wavelength have a profound effect on accuracy. Sensor calibration is the match between the spectral properties of the sensor and the R curve.
4. Sensor spectral errors will cause a predictable error in the R-value and consequent SATs value displayed on the monitor.
5. Optical functional testers mostly check light intensity to confirm functionality. They do not test for wavelength, which determines accuracy.
6. The testing process is incomplete and the users of pulse oximetry are falsely reassured, if the sensor is not tested for wavelength and accuracy.
Geoff,
I’m glad to see you’re still fighting the good fight.
At I see it, this issue is most important in neonates where oxygen toxicity is a real clinical concern. If the sensor shows in incorrect low reading you may be tempted to increase FiO2 – putting baby at risk. In all wards, if the reading is falsely high, the patient may be denied appropriate therapy, increasing length of stay and long-term outcomes.
I recommend all Biomeds understand this issue and implement appropriate measures to remove faulty SpO2 sensors from their inventory.
In a review I did (some years ago) we were surprised to find bad sensors from every manufacturer – the older the sensor the worse was the rate of failure. And if I recall correctly, the false low readings were more common than false high – this is related to LED wavelength drift and the SpO2 algorithm (but goes beyond a blog post to detail – read the papers cited above).
In my opinion it is far better you spend your time doing these checks than doing yet more meaningless and pointless electrical safety testing…
Oh, and it is true that you can’t “calibrate” SpO2 but you can verify accuracy using the appropriate tool (e.g. Lightman) but not by using the common SpO2 functional tester – they will not reveal inaccurate sensors – ever. They are good for finding the sensors with open circuit IR LEDs (Because you can’t see this failure visually as you can with the complimentary Red LED) but that is about all they do. To simulate desaturation, they assume an R curve and “filter” intensity of light received – it is a con.
I have personally done a breath-down test to verify this issue is real. A sensor that passed using a conventional tester was shown to be inaccurate during breath-down. We used also tested the sensor using the Lightman device and the Lightman device accurately predicted the error observed during the test. We had a second sensor on my other hand that the Lightman confirmed as good and this was accurate during the breath-down. THis testing was done without reps from Lightman being present.
Until this point I was a skeptic – not any more!
Patrick.
As a clinician it is disappointing, to say the least, that people ‘testing’ pulse oximetry are being encouraged to ignore accuracy. All simulators seem to be doing is pretending all sensors are perfect. However it is obvious that the wrong colour sensor will lead to “garbage in = garbage out” and the wrong SATs measurements. I think it is time that engineers moved forward to really test fully and not stay stuck with old technology and beliefs. Inaccurate measurements are affecting patients’ lives.
There are papers that have been published from research carried out by anaesthetists that would disagree with the statement ‘There are no accepted methods for verifying the correct calibration of a pulse oximeter other than human testing.’
1. Milner QJW, Mathews GR. An assessment of the accuracy of pulse oximeters. Anaesthesia 2012; 67: 369-401
2. Dugani S, Hodzovic I, Sindhaker S, et al. Evaluation of pulse oximeter sensor tester. Journal of Clinical Monitoring and Computing 2011; 25: 163-70
Andrew Clay’s article is welcome in that it highlights that pulse oximetry has become an accepted and recognised standard of patient care.
Where the article could be misleading is how it addresses the testing of pulse oximeters. The article notes that verification (type testing at design/factory stage) is carried out through controlled desaturation studies – not suitable for testing by biomeds.
The article goes on to quote Tobey Clarke’s recommendation of annual functional testings – leaving unclear how this can/should be carried out.
My concern with the article is that it does not really emphasise:
a. Limitations of simulators: that all that virtually all the spo2 simulators on the market can perform is indicating that the device is working, not that it is working accurately. Indeed, it could be argued that the simulator does little more than can be achieved by the biomed using the device on his/her own finger
b. The importance of accuracy, as other have commented on
c. That accuracy depends on the integrity of the sensor and in particular the wavelenghts of the light emmitted by the red and infrared LEDS. And that changes in the wavelenght from the design specification (poor batch, change in wavelenght with operating conditions) are likely to affect the accuracy
d. That methods are available to check the wavelenght of the LED lights sources
Best wishes
John
Its still not clear if the color pigments chanage due some reasons then what and how one has to rectifies it results
Sensor accuracy is dependent on placing LEDs of the correct wavelength in the sensor during production, and minimal changes in the spectral properties of the LEDs post production. This does not always happen and sensors can be wrong from new and can age.
The monitor is totally reliant on the red and IR wavelengths emitted by the sensor being correct. If these values are not correct the SATs values displayed on the monitor will not be correct. The SATs errors are greater at lower oxygen saturation values.
A short demonstration of a high reading sensor being tested can be viewed at: https://www.youtube.com/watch?v=pNxIAD_a_50
This error is mainly due to an error in the wavelength from the red LED. In use this sensor could lead to oxygen therapy and other important interventions being deferred or not given at all. No simulator or functional tester is capable of detecting this fault.
Patrick O’Meley’s findings in Australia agree with my experience. More sensors have a low reading bias than high. This is not only of obvious importance in managing premature babies but also for COPD/COAD patients in whom a higher level of oxygen may lead to metabolic acidosis due to Carbon Dioxide retention and subsequent morbidity (and possible mortality).
A high biased sensor will lead to clinical management that could cause hypoxia to be prolonged with associated tissue damage and outcomes – for the neonate this could be cerebral palsy. Adults are not immune to lack of oxygen with outcomes ranging from confusion, strokes and death.
None of the workflow proposed by Andrew Clay i.e. “checking the physical condition, performing electrical safety tests, performing any manufacturer-recommended preventative maintenance, conducting performance testing (including alarms and other specific tests), and, finally, documenting the testing results” includes any check of accuracy. The pulse oximeter will only function as the manufacturer intended if the attached sensor has the correct colour LEDs for the R-curve to which it is calibrated. Testing the sensor for accuracy must be an essential part of any testing regime, otherwise it is falsely (and dangerously) reassuring.
I recently conducted an audit looking at sensor accuracy of 227 pulse oximeter sensors over three different UK hospital trusts. I borrowed the Lightman from the UK based electrode company to run the accuracy tests. The Lightman is different from ‘functional’ testers as it measures the wavelength of the LED and compares this to the expected wavelength of the LED. If the LED puts out the wrong wavelength of light, a different amount of this light is absorbed by the blood, so a different amount of light passes through the finger to be read by the sensor, resulting in an inaccurate SpO2 measurement being calculated.
The results I found were really rather sobering.
In hospital one ~3% of the sensors were classed as unacceptable (unacceptable = error > +/- 3%).
In hospital two and three over one third of the sensors tested were classed as unacceptable.
Hospital one was unusual in that all of its sensors were of one make, which seems to have a much lower proportion of inaccurate sensors.
Scarily, the sensor with the highest bias of +8% was found on a patient in theatre. This patient’s true O2 saturation could have been significantly lower than that displayed on the monitor, with the potential for hypoxic damage.
The sensor with the largest low bias (-7%) was found in an anaesthetics room within the same hospital trust.
Depending which of these sensors was applied to the patient there could be a wide variety in what the sensors may read. For example:
– A patient with a true saturation of 90% could have a SpO2 value displayed of 94% (+/-3%) or 86% (+/-3%).
– With a SpO2 display of 90% the patient could have a true saturation of 84%(+/-3%) or 92%(+/-3%)
It is rather worrying that our hospitals have such a high proportion of inaccurate sensors! We need to start testing them for accuracy in addition to functionality!
Thank you all for feedback. This article touches on the limitations of testing pulse oximetry as a whole. We agree that the use of portable spectrometry could enhance fault detection of SPO2 sensors, and we welcome new innovation in this space. As with the medical technology itself, there are competing interests in ease of use, efficacy, and cost that are considered as new test technology is developed and deployed.
It should also be noted the IEC standard 80601-2-61 states, “There is today no accepted method of verifying the correct calibration of Pulse Oximeter Probe and Pulse Oximeter Monitor combination other than testing on human beings” (2014: line 2500).
Due to space limitations, this article does not discuss testing methods. We’d like to invite you download the full “Medical Equipment Quality Assurance” book referenced in this article to learn more about pulse oximetry test procedures and methods.
http://support.fluke.com/biomedical/Download/Asset/3276553_7150_ENG_A_W.PDF (Page 123)
Standards are often written with the benefit of hindsight available at the time they were written. Standards are the lowest common denominator that we should work to, but not be needlessly restricted by.
There appears to be some confusion over the process where the calibration data known as the R curve is established during the design phase, and the checking of accuracy of replacement sensors. The R curve is established by invasive desaturation studies where the results from a sensor of particular spectral properties are related to co-oximeter data. For replacement sensor to be accurate the emitter wavelengths must be a close match to the sensor used to establish the original R curve. As stated in the Pulse oximetry Standard (ISO 80601-2-61) – sensor accuracy is the match between the centre wavelengths emitted by the sensor and the calibration code used with that sensor.
The Fluke document referenced here is a long version of the list in the original article, and does not consider the impact and effect of replacement sensors. Sensor accuracy is important in pulse oximeter’s role in preventing adverse patient events. Users should be made aware that Healthcare Technology Management personnel following this referenced protocol do not test the correct match (accuracy) of the sensors, and that after an Annual Check of a monitor that the risk of a significant inaccuracy due to the sensor remains. Published data suggests that 30% of the sensors in use are unacceptable when tested. The cost benefit of appropriate clinical management of a patient and the cost risk of mismanagement needs to be remembered – this is a cost risk to a healthcare provider and not just the cost to an Engineering Department.
I read this article with a mixture of interest, disappointment, and concern.
It provides a succinct, easily read and understood description of the basic principles. Unfortunately it misleads the reader by asserting that accuracy is solely a function of design, faithfully reproduced at manufacture, and that subsequent verification is neither possible, nor needed.
In practice, and in respect of sensor manufacture, this in fact can be far from the reality. In addition, there seems to be evidence (from spectral testing) that suggests an ‘ageing’effect, particularly with IR emitters over time. Any deviation from the actual wavelengths used during controlled desaturation studies to derive the original R curve, whether at manufacture, or in subsequent use, will alter that R curve, and will bias the accuracy of the pulse oximeter. A difference of just a few nanometres in the Red emitter wavelength is sufficient to cause inaccuracy beyond the standards requirement of 3% (and without this error being further compounded by biological variability).
The article acknowledges that functional testers have a role; they can be an aid to verify that any given system is functioning; they cannot verify that it is providing accurate readings, because they have no means of analysing/verifying the emitted wavelengths. Functional testing is important to ensure that monitors are working correctly (alarms etc), but this does absolutely nothing for establishing confidence in accuracy.
It is disappointing that this article has not made this distinction, and further confuses the reader by requiring a verification of accuracy of 3% in the performance test sheet (The Medical Equipment QA book linked to this article, Page 124). Whilst the requirement is correct, the functional testers described cannot verify this.
Verified functionality does not imply performance accuracy.
The publications and studies alluded to in the replies to this article have collectively sampled a large number of sensors, both new and used. The many errors identified would suggest that we should be at least as concerned with measuring sensor wavelength accuracy, as with verifying instrument function, and yet there is no acknowledgment of this as a test requirement.
Pulse oximetry at its inception in the 1980’s was hailed as “the most significant technological advance ever made in monitoring the well being of patients during anaesthesia, recovery and critical care”.
Today, we are more dependent (in the UK at least) than ever upon accuracy, since our Oxygen therapy policies are now predicated upon treating to a pulse oximeter saturation reading for diagnosis and treatment.
If the essential requirements of the relevant standards are to be met (accuracy to within 3%), and patient safety to be maintained, then accurate RED/IR wavelengths are central to achieving this, and the sooner agreement is reached upon the methods of ensuring it, the better.
The technology of pulse oximeters appears relatively simple but is in fact somewhat complex. The spectral output of the red and the infra red LEDs in the sensor unit is central to the clinical accuracy of the device and where an initial verification of readings against blood measurements of oxygen saturation is undertaken for a highly specific spectral output of each LED. If during manufacture or device use the output spectra (particularly of the red LED) diverge from the ideal specification, then the indicated patient SpO2 value will not be accurate. This can give rise to significant errors where, for example, an indicated value of 95% may in fact be 88%. This type of error can only be detected with a specialist device – such as the Lightman – which measures the spectral output of each LED and can compare this with the core specification of the specific pulse oximeter device.
What are the moral and legal consequences when a patient is compromised by an inaccurate sensor?
Pulse oximeter sensor accuracy does matter. We see evidence of the impact of inaccurate plus oximeter sensors in mortality reviews where health care professionals have not known which sensor to believe.
What would happen if health care professionals and patients really knew what was going on with respect to inaccurate sensors?
Would simulator manufacturers that have failed to clearly advise that their products do not test sensor accuracy be liable?