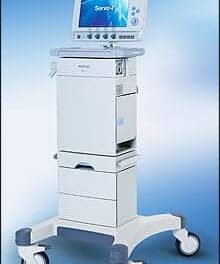
Synapse Biomedical announced that the U.S. FDA has granted premarket approval (PMA) of the NeuRx Diaphragm Pacing System (NeuRx DPS) for use in patients with spinal cord injuries who rely on mechanical ventilation.
The NeuRx DPS is an option for spinal cord injury (SCI) patients who prefer a more natural method of breathing. Rather than using positive pressure (forced air), NeuRx DPS provides negative pressure ventilation which mimics the body’s natural breath cycle.
Premarket approval is the most stringent type of device marketing application required by the U.S. FDA. More hospitals are expected to begin implementing the NeuRx DPS now that they no longer have to undergo the lengthy internal review and approval process required under the previous humanitarian device exemption, the company says.
“This is a very big deal for our community,” says Jen French, executive director of the North American Spinal Cord Injury Consortium, an advocacy group. “It will improve access and affordability for people living with spinal cord injuries and give them the chance to live life without the restrictions imposed by a ventilator.”
NeuRx DPS
The NeuRx DPS is a battery-powered device that delivers electrical stimulation via four percutaneous intramuscular electrodes implanted into the diaphragm with minimally invasive laparoscopy. It is intended for use in patients with stable SCI with stimulatable diaphragms, but who lack control of their diaphragms. The device is indicated to allow patients 18 years and older to breathe without the assistance of a mechanical ventilator for at least four continuous hours a day. Many patients have gone on to use the device up to 24 hours per day.
Pioneered by Raymond Onders, MD, Chair of Surgical Innovation at University Hospitals Cleveland Medical Center, and J. Thomas Mortimer, Emeritus Prof. of Biomedical Engineering at Case Western Reserve University (CWRU), NeuRx DPS was first implanted in a prospective clinical investigation in 2000 and has now been proven in over 2,500 patients worldwide.
“The new FDA approval is great news for patients,” says Onders. “Rapid weaning from mechanical ventilation allows for faster rehabilitation—giving patients a better chance of recovery, independence, and a new more normal way of life—while significantly reducing costs, freeing up ICU beds, and reducing clinical staff time with these patients.”
Actor Christopher Reeve was one of the first recipients of the system.
“Synapse Biomedical is dedicated to helping free people from mechanical ventilators. We are currently stimulating over 10,000,000 breaths per day for spinal cord injured patients who can’t breathe on their own around the world. With this FDA approval, we can make our diaphragm pacer available to many more patients who were previously unable to access a hospital administering our humanitarian device,” says Anthony Ignagni, CEO and founder of Synapse Biomedical.
Integrating Diaphragm Pacing into Standard of Care
The FDA approval should change the standard of care in treating SCI injuries, says P. Hunter Peckham, PhD, emeritus [rofessor of Biomedical Engineering at CWRU, and co-director, MetroHealth Rehabilitation Institute, MetroHealth System, a pioneer in neurostimulation and rehabilitation engineering.
“This life-altering technology should bring new freedom to thousands of people with SCI injuries,” he says.
The National Spinal Cord Injury Statistical Center estimates 17,900 new SCI cases each year. Since 2015, 59.8% of these patients have complete or incomplete tetraplegia, or paralysis of all four limbs, which will require some level of long-term or temporary mechanical ventilation.
Photo: Synapse Biomedical