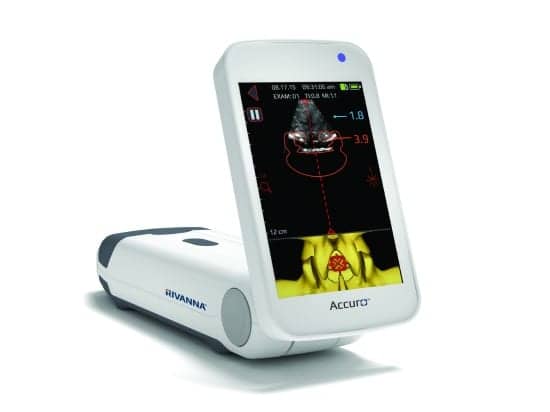
Ten months after receiving FDA 510(k) clearance to market Accuro in the United States, Rivanna Medical has introduced its handheld ultrasound device to the Middle Eastern market. Accuro, which helps guide spinal and epidural anesthesia with automated 3D navigation of the lumbar spine, provides precise identification of epidural location, depth, and trajectory. It also enables ultrasonic imaging of abdominal, musculoskeletal, cardiac, and peripheral vascular anatomies.
Will Mauldin, Rivanna Medical’s CEO, says the decision to sell the device in the Middle East was highly strategic. In fact, the company has even forged partnerships with international distributors to get the product to market faster.
“These partnerships in Saudi Arabia and the United Arab Emirates, respectively, demonstrate the far-reaching significance and opportunity of our easy-to-use Accuro device,” Mauldin says. “The Middle East expansion, combined with the level of regional market adoption we have seen for Accuro, is a strong indication of Rivanna Medical’s continued success on all fronts.”
The Accuro platform is designed to take the guesswork out of spinal procedures by providing automated navigation to an anatomical target—a major advantage for patients, Maudlin says. “If we can increase the efficiency of epidural and spinal anesthesia with fewer needle sticks, while reducing complications and costs, then this is clearly an improvement for both the anesthesia community and the patient,” he adds.
For more information about this device, visit Rivanna Medical.




Great, we would like to see live demonstrations in our hospital ,Department of Anesthesia,Al Amiri Hospital,Moh in kuwait