The new portable systems are designed for a range of clinical applications and feature artificial intelligence and advanced imaging functionalities.
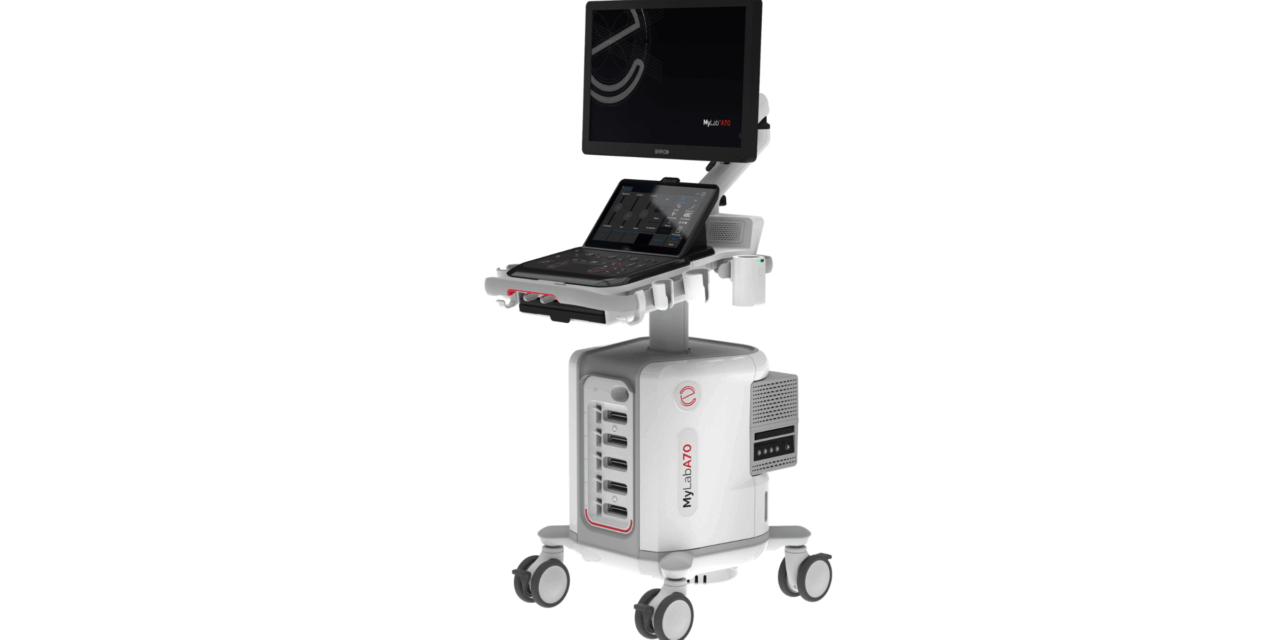
Esaote has received US Food and Drug Administration approval for its MyLab A50 and MyLab A70 ultrasound systems, clearing the devices for distribution in the US. The medical imaging company announced that the approval confirms the systems meet required standards for safety and clinical performance.
The MyLab A50 and MyLab A70 systems are engineered for flexibility in various clinical settings. Designed to be portable, the models feature compact sizes and battery operation to support the mobility needs of healthcare practitioners.
“The new A-series emphasizes user experience with a diverse range of interface options, including both a conventional and touch control panel. The devices boast an intuitive, easy-to-clean design that allows clinicians to operate efficiently and confidently,” says Thomas Will, director of ultrasound sales at Esaote North America, in a release.
The systems are equipped to handle numerous clinical applications, from routine diagnostics to advanced imaging. They support ultrasound functionalities such as liver elastography and attenuation imaging, along with cardiology tools like strain analysis, for detailed assessments. According to the company, the platforms use artificial intelligence and other imaging advancements to help providers make precise diagnoses.
Photo caption: The Esaote MyLab A70 ultrasound system
Photo provided: Esaote