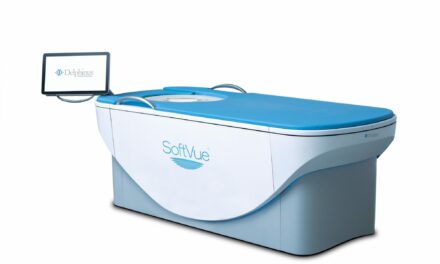
The investigational system uses X-ray diffraction and AI to analyze the molecular composition of tissue, aiming to reduce unnecessary biopsies.
Calidar, Inc has announced the successful imaging of the first patient with its investigational 4D Mammography system, initiating a first-in-human clinical study. The system combines X-ray diffraction technology with AI to measure molecular-level signals of disease, with the goal of improving diagnostic accuracy in breast imaging.
The technology addresses challenges in breast cancer detection, where a high number of biopsies are performed on benign tissue. According to the company, approximately 1.5 million breast biopsies are performed annually in the US, with up to 80% later diagnosed as benign, contributing to over $6 billion in yearly healthcare costs and increasing workloads for radiologists and pathologists.
Calidar’s system measures how X-rays scatter at the molecular level, a process known as X-ray diffraction. This produces a structural signature that reflects the internal composition of breast tissue. Unlike traditional X-rays, which analyze shape and density, this method is designed to reveal what the tissue is made of. The company reports that prior ex vivo studies of breast tissue showed this technique enabled classification of cancerous and benign samples with four times the precision of conventional imaging methods.
“X-ray diffraction has unlocked some of the most iconic achievements in science—from discovering the structure of DNA to revealing the composition of another world on the Mars rover—and now we are bringing its power into the clinic to look inside the human body in a completely new way,” says Dr Stefan Stryker, CEO of Calidar, in a release. “Our 4D Mammography system brings this capability to the challenge of breast cancer diagnostics, where high-precision, noninvasive imaging tools are urgently needed.”
Clinical Study and Regulatory Status
The first-in-human study is being conducted in collaboration with Baptist Health Hardin in Elizabethtown, KY, and is led by Principal Investigator Dr Craig Kamen. The study will assess the system’s ability to distinguish between healthy tissue and breast cancer and will compare its performance to existing mammogram devices.
“We are excited to collaborate on this next-generation research and contribute to the development of technology that could meaningfully enhance our capabilities for diagnosing breast cancer,” says Dr Kamen, in a release.
The current study is focused on diagnostic applications for patients who have findings that require further evaluation. The company plans future studies to explore its use in breast cancer screening.
The 4D Mammography system is an investigational device and has not been cleared or approved by the US Food and Drug Administration. It is not available for commercial sale and is limited to investigational use in the US.
Photo caption: Calidar’s patented hardware, licensed from Duke University, that enables X-ray diffraction imaging in 4D Mammography.
Photo credit: Calidar