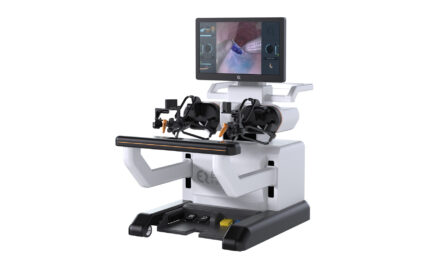
Nano-X Imaging Ltd (Nanox), a medical imaging technology company, has received a 510(k) clearance from the U.S. FDA to market the multi-source Nanox.ARC, including the Nanox.CLOUD, its accompanying cloud-based infrastructure. Nanox.ARC is a stationary X-ray system intended to produce tomographic images of the human musculoskeletal system adjunctive to conventional radiography on adult patients. Representing a major advancement in X-ray technology, Nanox.ARC is a multi-source digital 3D tomosynthesis system that utilizes novel, cold cathode X-ray tubes, which the company intends to offer using an innovative pay-per-scan business model.
The FDA cleared Nanox.ARC for use in professional healthcare facilities or radiological environments, such as hospitals, clinics, imaging centers, and other medical practices by trained radiographers, radiologists, and physicians, and has the potential to increase availability to medical imaging around the world, once approved by local regulatory authorities and deployed at scale.
The company received 510(k) clearance to market its single-source X-ray device, known as the Nanox Cart X-Ray System, in April 2021. The multi-source Nanox.ARC has the ability to reconstruct a series of 2D projection images into a stack of tomograms (or slices) of the imaged object, forming a 3D visualization. This visualization reduces the effect of overlying structures and provides in-depth information on structures of interest.
“Today’s milestone is a significant achievement as part of our commitment to make state-of-the-art medical imaging technology available for use in a wide array of professional healthcare facilities and other medical practices,” says Erez Meltzer, chief executive officer of Nanox. “Our vision is that Nanox’s innovative technology and approach not only have the potential to increase access to medical imaging, but also to shift healthcare from reactive to proactive—enabling early detection and prevention of diseases.”
Following this clearance, Nanox will continue to work with the FDA to pursue additional regulatory clearances and intends to expand clinical indications. The U.S. regulatory clearance also paves the way for Nanox.ARC to be approved in other countries that are FDA-clearance-based markets. Other applications will be available in other markets per local regulatory approvals.
Medical imaging systems are an important early detection tool that are key to initiating early treatment, improving health outcomes, and ultimately saving lives. Approximately two-thirds of the world’s population does not have access to medical imaging systems, according to the World Health Organization (WHO), while many people with access face substantial wait times for scanning, potentially delaying diagnoses. By introducing an innovative pay-per-scan business model, Nanox offers an opportunity to help expand these resources to more healthcare settings.
While access to medical imaging is relatively high in the U.S. compared to many other countries, medical imaging can still be limited in certain areas, particularly in rural or low-income areas where facilities and equipment may be scarce. For example, compared with patients at urban hospitals, emergency department patients in rural hospitals are 7% less likely to receive advanced imaging, while patients at critical access hospitals are 18% less likely to have access to advanced imaging.
“The FDA clearance of Nanox.ARC represents an important breakthrough and represents an opportunity to increase the availability and accessibility of medical imaging in the United States and worldwide,” says Geoffrey Rubin, MD, MBA, professor and chairman of the Department of Medical Imaging at the University of Arizona and a member of Nanox’s advisory board. “Medical imaging is essential for detecting, diagnosing, and managing disease, guiding treatment decisions for improved health outcomes. Nanox.ARC has the potential to be a cost-effective and scalable imaging solution in healthcare settings that would otherwise be unable to deploy traditional medical imaging equipment.”