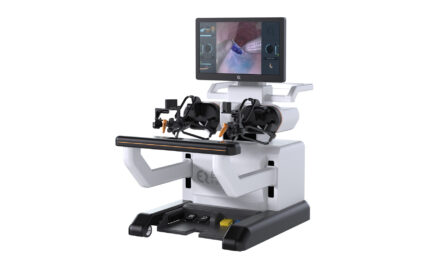
GE HealthCare announced US FDA 510(k) clearance of its Carescape Canvas patient monitoring platform for precise, flexible patient care. Carescape Canvas and Carescape ONE are part of an adaptable ecosystem that can easily scale up and scale down monitoring capabilities based on the acuity of each individual patient, delivered on a standardized platform designed to drive enterprise efficiency and ease of service.
“As patient acuity and disease trends change rapidly, adaptability in the hospital setting is becoming more important. We look for ways to improve the care experience by delivering quality clinical measurements where and when they are needed, giving care providers the information they need to make the best possible clinical decisions,” says Dr Roland Schrewe, head of PICU, University of Frankfurt. “GE HealthCare’s Carescape Canvas provides a precise, standardized, and flexible patient monitoring solution that adapts to patient needs with the ease of switching out a cable.”
Carescape Canvas is a FlexAcutity solution equipped with smart parameter technology that can be used for patients at all levels of acuity. This monitoring platform offers flexibility, standardization, and ease of use by leveraging micromodules that connect with standardized medical USB technology—simply adding or disconnecting cables equipped for various configurations enables more efficient care based on patient care needs. It is also equipped with flexible, easy-to-configure software and interchangeable frames for advanced parameters, enabling monitoring devices to be quickly redeployed across the hospital enterprise as needed.
“This new monitoring ecosystem has the potential for a hospital to have one single unified approach to patient monitoring that can be easily tailored for each patient,” says Neal Sandy, general manager, monitoring solutions, GE HealthCare. “GE HealthCare is committed to offering flexible solutions that enable care teams to focus on the patient, not the technology. Carescape Canvas’ innovative approach, where software and patient parameters can change in a very nimble manner, enable a standardized ecosystem that can adapt to changing healthcare needs.”
The Carescape Canvas bedside monitor was developed and tested with the rest of GE HealthCare’s ecosystem, not only with the current devices available, but also with prior versions that are used across the 100 million patients monitored by GE HealthCare devices each year. This backward compatibility allows healthcare systems to upgrade to the latest capabilities at their pace. Carescape Canvas was also developed with sustainability in mind, with a 53% reduction in packaging volume, 48% reduction in packaging weight compared to previous generation monitor, and a 25% reduction in energy consumption compared to previous models. In addition, Carescape Canvas monitors made at GE HealthCare’s Helsinki site are manufactured by using 100% renewable wind electricity.
Carescape Canvas was recently awarded the world-renowned, iF Design Award 2023. The jury prize honors design achievements in all disciplines and recognized Carescape Canvas for product design in medicine/health.
Carescape Canvas has been approved for use in the EU since June 2022 and is now cleared for use in the United States.