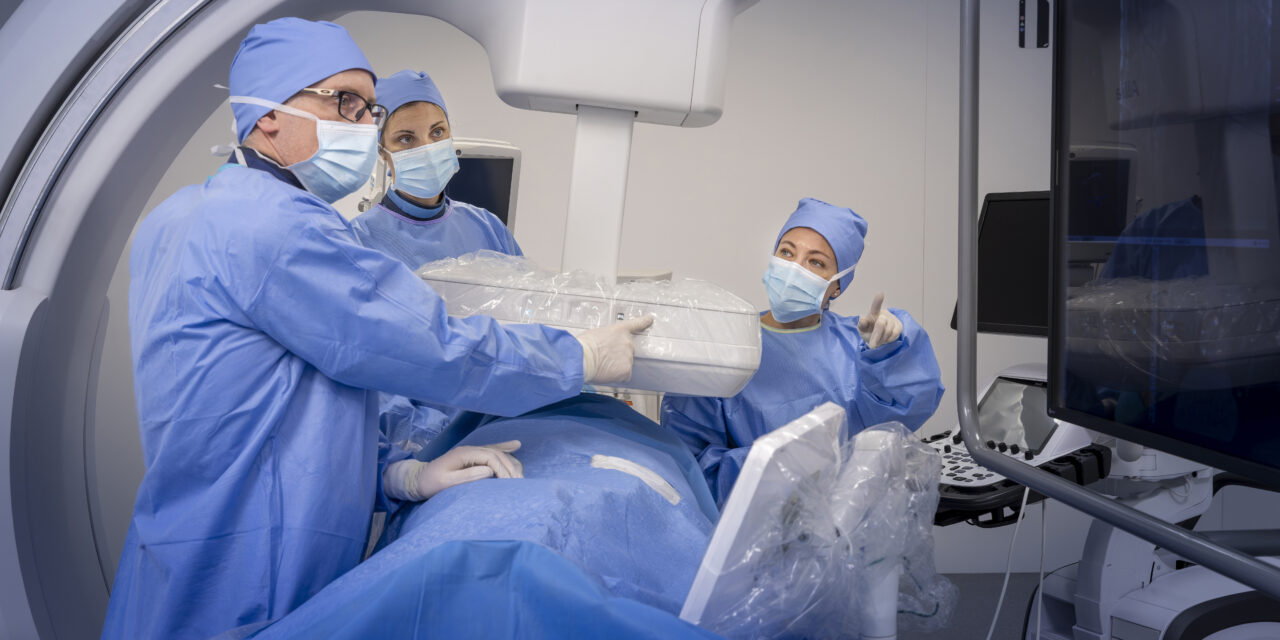
The mobile C-arm system features AI-powered imaging tools and cable-free design for interventional procedures.
GE HealthCare has received US Food and Drug Administration 510(k) clearance and CE marking for its Allia Moveo interventional imaging system, with installations now underway at medical centers in France and the United States.
The mobile C-arm platform combines cone-beam computed tomography capabilities with AI-powered imaging tools designed for cardiovascular, vascular, and surgical procedures. The system features a cable-free design and compact footprint intended to address space constraints in traditional interventional suites.
Hôpital Marie-Lannelongue in France completed the initial global installation of Allia Moveo. The facility specializes in thoracic, vascular, and heart surgery and serves as a national reference center for complex aortic pathologies.
“My initial experience with Allia Moveo has been incredible; it’s a real game-changer. With Allia Moveo, we had this opportunity to position the system very quickly in any working position that will adapt the best to us,” says Stephan Haulon, MD, PhD, vascular surgeon and head of aortic center and vascular surgery at Hôpital Marie-Lannelongue, in a release. “One of the major milestones of this new system is having access to the reconstruction of the cone beam CT with CleaRecon DL, which is AI-driven and gives you much better image quality.”
AI-Enhanced Imaging Features
The system incorporates several advanced imaging technologies, including CleaRecon DL, an AI-driven tool that removes streak artifacts caused by arterial blood flow to deliver clearer images. Additional features include Motion Freeze technology to reduce respiratory motion artifacts and Metallic Artifact Reduction to reveal anatomic details obscured by metal implants.
The platform also includes augmented guidance solutions including that ASSIST advanced clinical applications portfolio for procedure guidance and maintains compatibility with other GE HealthCare and third-party systems. The system operates at noise levels described as quieter than normal conversation.
“With Allia Moveo, we’re elevating the clinician experience, removing complexity, accelerating workflow, and enabling greater control during procedures,” says Arnaud Marie, general manager of interventional solutions at GE HealthCare, in a release.
US Installation Completed
Following the French installation, Baylor St. Luke’s Medical Center in Houston completed the initial US installation of the system. The facility is part of the CommonSpirit health system.
“We are honored to be the initial hospital in the United States to install Allia Moveo, and proud to help advance the next generation of interventional care,” says Brad Lembke, MD, president of Baylor St. Luke’s Medical Center, in a release. “This innovative platform enhances how our clinicians navigate complex minimally invasive procedures by improving mobility, image clarity, and workflow efficiency.”
The system features a wide-bore design and table panning capabilities intended to accommodate patients of varying body sizes. The ergonomic design aims to maintain clinician comfort and access throughout procedures.
GE HealthCare unveiled the Allia platform at the Radiological Society of North America’s 2025 Annual Meeting. Product availability varies by region.
Photo caption: Allia Moveo
Photo credit: GE HealthCare