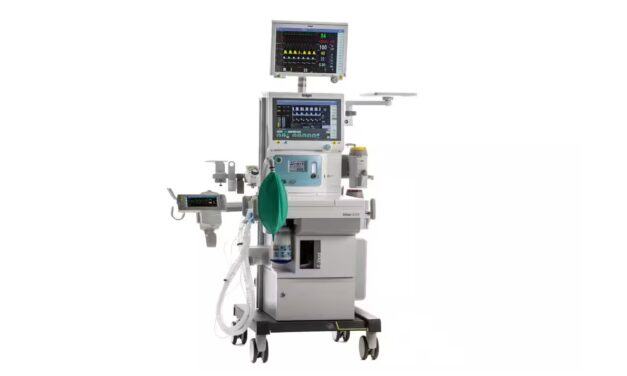
Vizient Names Dräger Anesthesia Machines Innovative Technology
Dräger’s Atlan A350/A350XL Anesthesia Machines received an Innovative Technology designation from Vizient for their electronic piston ventilator and advanced safety features designed to reduce anesthetic agent consumption.
Read More