In studying for your CBET exam, you may have encountered the subject of spectrophotometry and how it can be used to determine chemical substances in a solution, thanks to Beer’s Law. As you may recall, Beer’s law states that the amount of light absorbed by a medium is proportional to the concentration of the absorbing material or solute present. The amount of light the material absorbs depends upon the wavelength or color of the light.
In studying for your CBET exam, you may have encountered the subject of spectrophotometry and how it can be used to determine chemical substances in a solution, thanks to Beer’s Law. As you may recall, Beer’s law states that the amount of light absorbed by a medium is proportional to the concentration of the absorbing material or solute present. The amount of light the material absorbs depends upon the wavelength or color of the light.
The principles of spectrophotometry enable us to perform cell counting by light transmittance. One advantage of using this technology for cell counting is that a sample size as small as 1 µL can be used. The disadvantage of using this technology for cell counting is that it does not directly count the cells. Instead, one must extrapolate cell count or biomass count by the light transmittance or turbidity displayed by the sample. (The process of measuring the loss of light intensity is known as turbidimetry.)
The simplest way to do a cell count is to count the cells manually under a microscope. This method uses a microscope slide prepared with the sample to be counted. The sample is placed in specialized slides with grid patterns. Knowing the percentage of sample dilution and the size and the depth of the sample grid pattern, one can determine the cell count by manually counting the visualized cells. This manual process is slow and susceptible to human error, especially if many samples are being counted.
Coulter Counters
Today, most cell counters use the Coulter Principle to determine cell count. The Coulter Principle states that the impedance of a cell is proportional to the volume of the particle or cell. Thus, a Coulter counter can determine how many cells are in a sample and distinguish between cell volumes. Cells by themselves are not very good conductors, so they show up as high impedance to electrical current. This fact is used to determine cell count.
A Coulter counter is used heavily in the hematology department within a hospital laboratory for complete blood counts (CBCs), which are routinely ordered for diagnostic purposes. A CBC measures several components and features of your blood, including red and white blood cells, hemoglobin, hematocrit, and platelets.
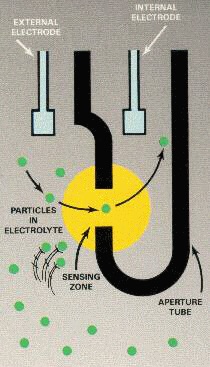
The lab can determine the amount of red blood cells and white blood cells in the sample thanks to the fact the Coulter counter can determine not only the presence of a cell but also the volume of the cell. The blood sample is suspended in an electrolyte solution that changes the electrical characteristics of the sample. Remember, cells show high impedance to electrical current, while electrolytes show very little impedance to electrical current.
As the sample is introduced between two electrodes through microchannels, the amount of current flowing from electrode to electrode is a function of the impedance between the two electrodes. Thus, every time a cell comes between the electrodes, there is a decrease in current on account of the impedance the cell displays. A Coulter counter determines the size of the cell by its impedance signature, as the Coulter Principle states the impedance of a cell is proportional to its volume. So, every cell that passes between the electrodes will produce a decrease in current. The amount of decrease in the current is a function of the volume of the cell.
Each red blood cell, or erythrocyte, has a volume of about 90 femtoliters (fL), or approximately a volume of 10–15 L. White blood cells, or leukocytes, have a volume approximately between 247 and 534 fL. This is a large enough difference in volume, and therefore impedance, that a Coulter counter can determine which type of cell has been counted. Figure 1 illustrates this concept.
Aperture Versus Flow Cell
Cell counters usually come in two varieties: an aperture format and a flow cell format. The flow cell format uses electrodes at each end of the flow channel. This allows for continuous sample analysis. By contrast, the aperture format is only for single-batch analysis.
For proper analysis of the sample, sufficient diluting of the sample must take place. This is because only one cell at a time can flow between the two electrodes. The dilution can be accomplished by using precision milled microchannels, which introduce the sample or by a sheath fluid, as shown in Figure 2.
These techniques ensure that only one cell is counted at a
time, and not a group of cells. When groups of cells are counted as one cell, this is regarded as a cell count abnormality, called “coincidence.”
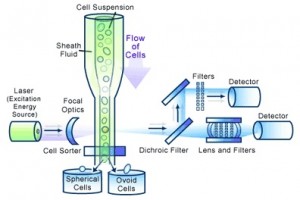
The most sophisticated and expensive cell counters use a technology referred to as flow cytometry. Figure 2 shows a diagram of flow cytometry.
This technology does not use current to determine cell count. Instead, flow cytometry uses a highly focused laser light that hits each cell as it passes through the beam. Each cell reflects a portion of the light and is picked up by light detectors after passing through dichroic filters (which function like a prism).
This technology can also determine cell shape as well as the internal and external structures of the cells. It can analyze thousands of particles a second. A very expensive technology, flow cytometry equipment is rarely, if ever, purchased to perform cell counting alone.
As always, I hope you find the information presented here helpful in your understanding of medical instrumentation and useful in your preparation for certification.
Review Questions
1) Spectrophotometry measurements are determined by which law?
a) Ohm’s Law
b) Coulter Principle Law
c) Electromagnetic Law
d) Beer’s Law
2) Red blood cells have a volume of approximately _____.
a) 247 cubic microns
b) 247 fL
c) 90 fL
d) 534 fL
3) Turbidity in cell counting refers to ____.
a) Nonlaminar flow
b) Error in measurement
c) Loss of light intensity
d) Coincidence
4) Cell counters are great at producing cell counts for CBC tests. In what area of the lab would you most likely find CBC testing performed?
a) Hematology
b) Pathology
c) Blood Bank
d) Virology
John Noblitt, MAEd, CBET, is the BMET program director at Caldwell Community College and Technical Institute, Hudson, NC. For more information, contact editorial director John Bethune at [email protected].
Answers: 1—D, 2—C, 3—C, 4—A