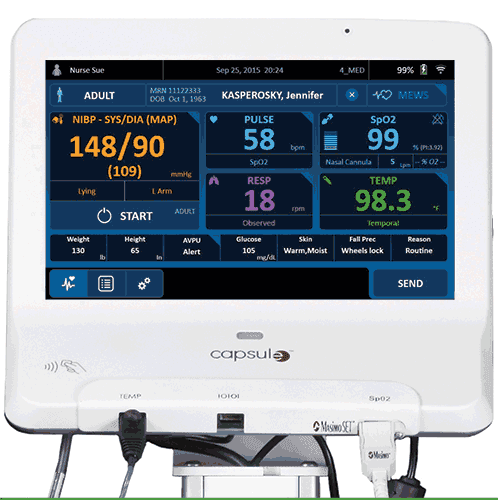
 Capsule, a subsidiary of Qualcomm Life Inc, has received FDA 510(k) clearance of its SmartLinx Vitals Plus, a patient monitoring system that integrates vital signs monitoring and clinical documentation into one scalable platform.
Capsule, a subsidiary of Qualcomm Life Inc, has received FDA 510(k) clearance of its SmartLinx Vitals Plus, a patient monitoring system that integrates vital signs monitoring and clinical documentation into one scalable platform.
The system, part of Capsule’s SmartLinx Medical Device Information System, is designed as an alternative to traditional low-acuity monitors. It is intended to provide new functionality in a single platform that allows hospitals and other healthcare facilities to streamline user authentication, patient identification, vitals measurement, and clinical documentation by integrating vital signs modules and components directly to the SmartLinx Neuron 2 mobile clinical computer.
“Today’s patient bedside is crowded with technology used to capture, chart and review the complete set of patient vitals, and we see tremendous opportunity in technological convergence—the ability to combine these multiple components into a single device or ‘all-in-one’ solution requiring one workflow,” said Kevin Phillips, vice president, marketing and product management, Capsule. “In SmartLinx Vitals Plus, we developed a fully integrated mobile system that streamlines the patient monitoring and clinical documentation process through a clinician-friendly, flexible workflow—complete with an early warning scoring system—that presents vitals data all on one screen, right at the point of care.”
According to Capsule, the SmartLinx Vitals Plus is designed to offer healthcare facilities an alternative to conventional monitoring as they retire older vital signs monitors. This transition typically requires a connectivity solution to automate charting and integration with the hospital electronic medical record. SmartLinx Vitals Plus is designed to convert a clinical IT device into a biomedical device to support healthcare organizations that are converging their IT and biomed/clinical engineering organizations.
The company reports that it is planning limited deployment of SmartLinx Vitals Plus to key customers throughout the fourth quarter of 2015, with wider distribution beginning in 2016. For more information about SmartLinx Vitals Plus, visit the Capsule website.