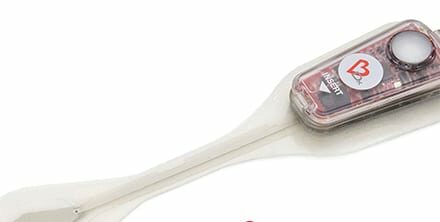
VERO Biotech, a commercial-stage healthcare business dedicated to neonatal intensive care and the acute care hospital community, received FDA approval of the newest generation of its tankless inhaled nitric oxide (iNO) delivery system.
The Third Generation GENOSYL Delivery System has new features that are expected to deliver three key benefits for patients, clinicians, and providers. This includes:
- Faster dosing, enabled by an adaptive sensor and automated cassette activation that accelerate time to achieve the desired dose.
- Simpler workflow, as clinicians can now work from one console. The new delivery system has a dual cassette bay within each console, and transitions cassettes automatically. Previously, with a single cassette, clinicians needed to transition to a second console.
- Operational efficiency, facilitated by an improved user interface and smaller, lighter disposable cassettes that alleviate storage constraints within hospitals.
Inhaled Nitric Oxide dilates pulmonary blood vessels and may be used to improve oxygenation in neonates with hypoxic respiratory failure and pulmonary hypertension.
“The enhancements to the GENOSYL Delivery System Console will help me to work more efficiently,” says Denise Lauderbaugh, MPH, BSRC, RRT-NPS and clinical practice wpecialist, Rady Children’s Hospital, San Diego, CA. “It has two cassettes in one console, and they are even smaller than before; it automatically activates and transitions to the second cassette when the first one is depleted; and I no longer have to transition from a primary to a standby console. I can care for my patients through the operation of just one console.”
GENOSYL DS is the first tankless inhaled nitric oxide delivery system approved by the U.S. FDA, the company says.
“The continuous innovation of the GENOSYL Delivery System represents our commitment to neonatal intensive care and the acute care hospital community in providing solutions to the challenges they face,” says Brent V. Furse, CEO and president of VERO Biotech. “We are grateful for the partnership and support we received with the launch of our innovative tankless GENOSYL Delivery System and this collaboration that has and will allow VERO Biotech to continue to expand on its mission to save lives, alleviate suffering and improve the health economics of acute care.”
Unlike tank-based systems, GENOSYL DS generates iNO at the bedside using a small disposable cassette. This eliminates the need for hospitals to manage large, cumbersome tanks and helps to simplify clinical workflow.
Featured image: VERO Biotech Receives FDA Approval of its Third Generation Tankless Inhaled Nitric Oxide Delivery System. Photo: VERO Biotech