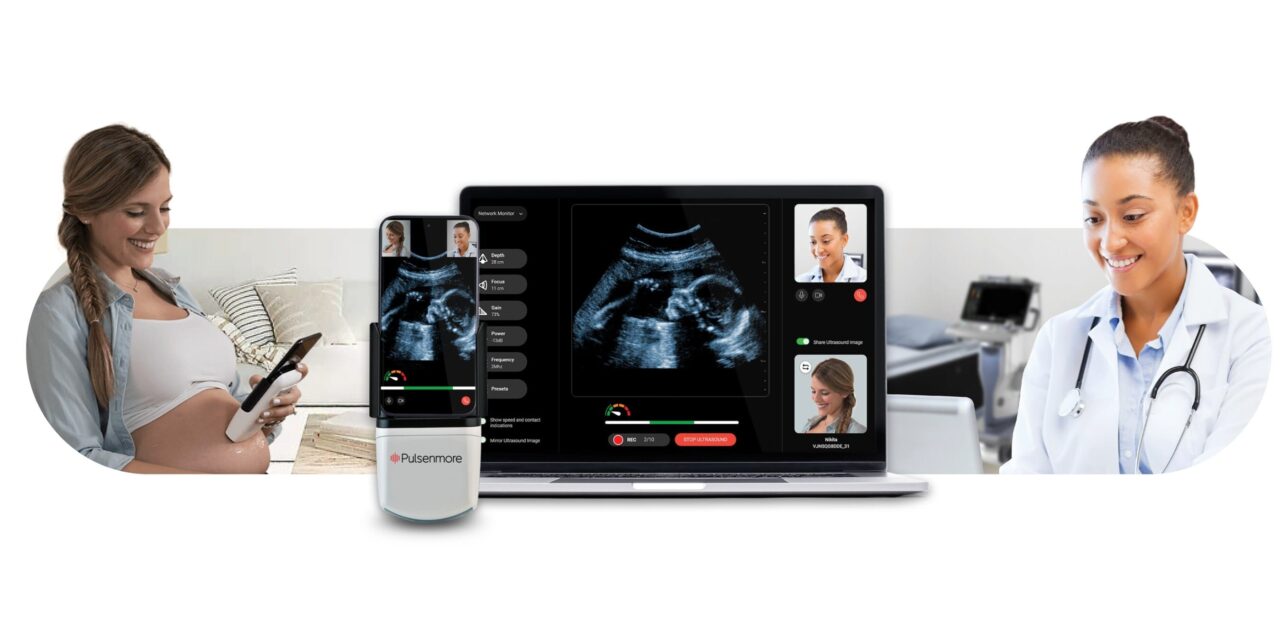
The platform allows expectant mothers to perform guided scans at home, with physicians reviewing images remotely through a dedicated platform.
Pulsenmore Ltd announced that the US Food and Drug Administration (FDA) has granted De Novo marketing authorization for the Pulsenmore ES, a home-use prenatal ultrasound platform that enables expectant mothers to perform guided scans at home interpreted by remote physicians.
This authorization introduces to the United States a care model that connects expectant mothers and physicians through guided imaging and digital review.
The Pulsenmore ES integrates at-home ultrasound imaging into physician-led prenatal care. The Pulsenmore cradle connects to the patient’s smartphone and guides her step-by-step via the Pulsenmore app. Captured video clips are securely transmitted to physicians for review and interpretation on a dedicated dashboard. Pulsenmore’s US clinical validation was achieved through a multi-center trial conducted at four leading academic and clinical institutions.
“This is a long-awaited leap forward in obstetrical care,” says Dr Wulf Utian, an expert in women’s health, in a release. “By reducing unnecessary visits, easing maternal anxiety, and improving triage, Pulsenmore’s innovation benefits patients, clinicians, and healthcare systems alike.”
The Pulsenmore ES does not replace in-clinic diagnostic or anatomical ultrasound examinations, but complements existing workflows in alignment with the American College of Obstetrics and Gynecology Guidance for Tailored Prenatal Care Delivery for Pregnant Individuals, notes a release from the company.
Pulsenmore’s home ultrasound platform is already in use across health systems in Israel, Europe, Brazil, and Australia. Pulsenmore is preparing for a phased US launch in collaboration with clinical institutions in early 2026.
“Pulsenmore’s solution bridges distance and capacity barriers, enabling physicians to stay connected to their patients throughout pregnancy,” says Dr Elazar Sonnenschein, Pulsenmore’s CEO and founder, in a release. “Pulsenmore ES brings connected, clinician-led ultrasound directly into the home-making prenatal care more accessible and equitable.”
Access to the device operation must be granted by prescribing physicians.
Photo caption: Pulsenmore ES
Photo credit: Pulsenmore