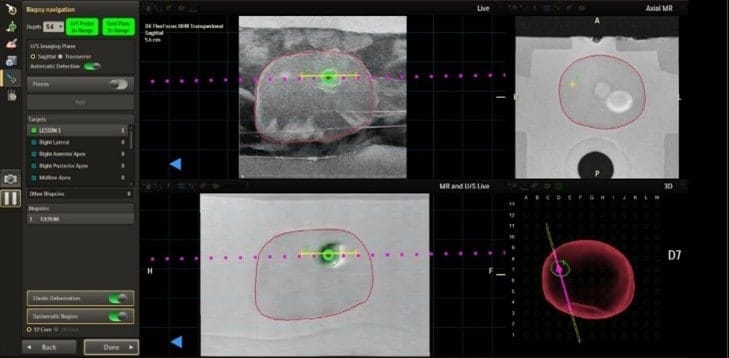
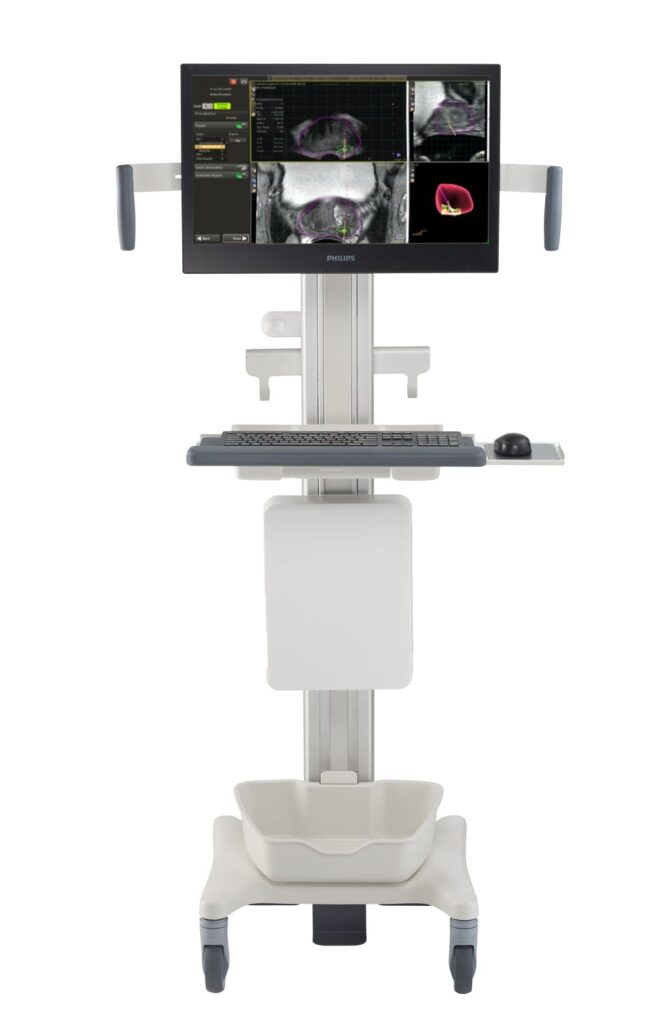
The newly cleared platform combines MR and ultrasound imaging to support real-time guidance for focal therapy and improve clinical accuracy.
Royal Philips announced it has received US Food and Drug Administration (FDA) 510(k) clearance for the latest UroNav version for minimally invasive focal therapy procedures in prostate cancer care.
As an image fusion system, UroNav integrates pre-procedural imaging, such as magnetic resonance (MR) imaging, with real-time intra-procedural imaging from ultrasound (US) systems. This combination aims to enhance the precision and accuracy of therapeutic procedures, providing clinicians with a comprehensive view of the targeted area.
“Philips’ integrated focal therapy platform unifies imaging, biopsy pathology, treatment planning, and 3D imaging guidance with MR US fusion, giving clinicians end-to-end efficiency and control,” says Ardeshir Rastinehad, DO, vice chair of urology at Lenox Hill Hospital and System director of Prostate Cancer at Northwell Health, in a release. “With fused imaging and real-time ablation guidance in one place, we can personalize therapy with greater accuracy and spare patients the unnecessary side effects of traditional treatments.”
Research has shown a 30% improvement in high-risk prostate cancer diagnosis using fusion biopsy compared to the standard biopsy.
Additionally, the system includes a new annotation workflow that works in tandem with DynaCAD Urology to support focal therapy planning, delivery, and review.
“We’re helping clinicians deliver more precise prostate cancer care by streamlining complex workflows and delivering the insights they need to support precise diagnosis and expand options for minimally invasive treatments,” says Martijn Hartjes, business leader, clinical informatics at Philips, in a release. “Our goal is to equip clinicians with the clinical tools required so they can deliver better care for more patients.”
In addition to clinical functionality, Philips UroNav offers enhanced compatibility with ultrasound devices and needle guides, upgraded privacy and security protections, and seamless integration with Philips DynaCAD systems for radiology and urology.
Photo caption: UroNav with advanced annotation
Photo credit: Royal Philips