Newly cleared technologies aim to improve image quality, streamline workflows, and support a range of clinical applications across nuclear medicine.
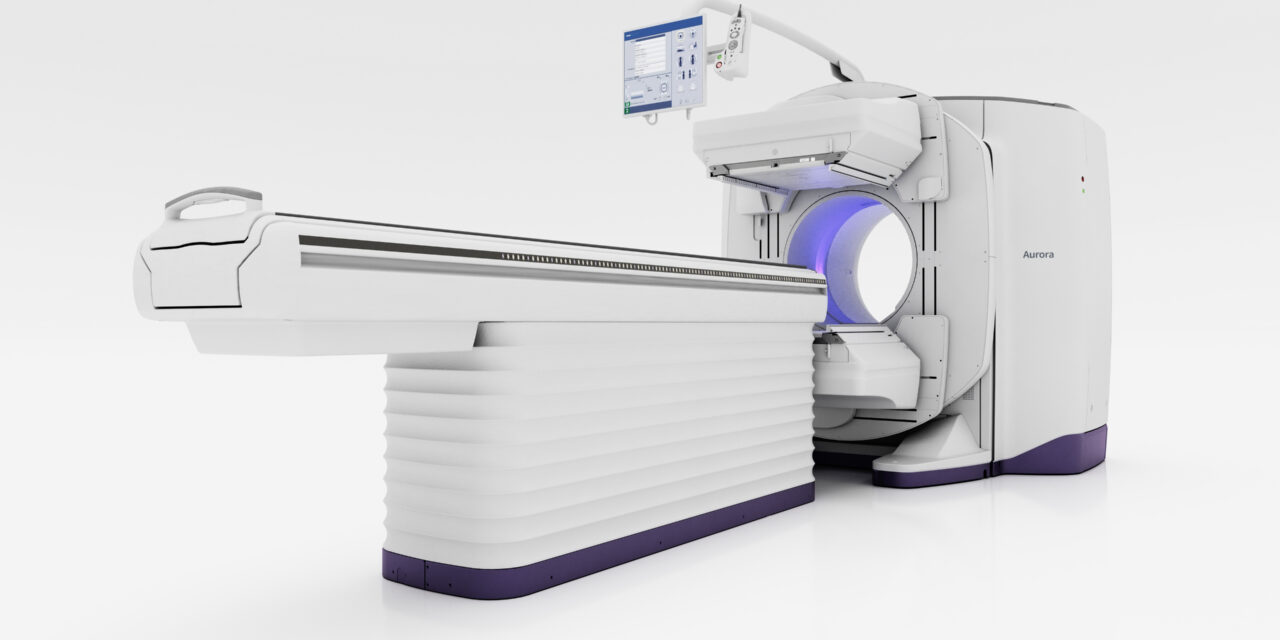
GE HealthCare announced that the US Food and Drug Administration (FDA) granted 510(k) clearance of Aurora, a new dual-head SPECT/CT system, and Clarify DL, its AI-powered deep-learning image reconstruction technology.
Aurora is designed to provide imaging precision, streamline workflows, and enable an improved patient experience. Its 40 mm detector—twice the detector coverage compared to CTs of other hybrid systems—and 128-slice plus intelligent imaging capabilities help support a range of clinical applications, from cardiology to oncology and neurology. Furthermore, Aurora’s available 5/8-inch crystal NaI detectors make it a suitable system for a range of clinical radiopharmaceuticals, including those used in the field of theranostics, according to a release from the company.
Designed to build on this performance, Clarify DL offers image quality without having to increase injected dose or scan time. Leveraging deep learning technology, Clarify DL works to enhance bone SPECT image quality performance with clear, accurate, and effortless imaging, according to a release from GE HealthCare. In a clinical evaluation, Clarify DL’s image resolution was rated as better in 98% of the exams. Unlike traditional noise reduction techniques that may compromise contrast and resolution, Clarify DL is designed to optimize reconstruction without sacrificing image quality.
[RELATED: GE HealthCare Unveils New CT System to Support Growing Demand for Cardiac Scans]
Additionally, Aurora is equipped with “effortless workflow” solutions to help ease technologists’ work from pre-scan to post-scan with various automation and design innovations, as well as help enhance patient comfort, including those with high BMI.
“Aurora and Clarify DL are powerful reflections of GE HealthCare’s ongoing investment in next-generation imaging solutions that empower clinicians to practice precision medicine and make more informed decisions,” says Jean-Luc Procaccini, president and CEO of molecular imaging and computed tomography at GE HealthCare, in a release. “By providing diagnostic precision while enabling improved workflow, these nuclear medicine technologies allow clinicians to deliver effective, patient-centered care—ultimately helping drive better outcomes.”
University Hospitals in Cleveland, Ohio, has become the first healthcare system in the United States to install this technology.
“We are thrilled to be the first in the United States to adopt this incredibly impressive technology,” says Donna Plecha, MD, chair of the department of radiology, radiologist-in-chief, and Ida and Irwin Haber and Wei-Shen Chin, MD Chair in Radiology at University Hospitals, in a release. “Aurora’s seamless integration of SPECT and CT components will allow us to perform comprehensive, high-quality diagnostic exams in a single session, while its support of Clarify DL deep-learning image reconstruction enables enhanced image quality performance.”
Photo caption: Aurora system
Photo credit: GE HealthCare