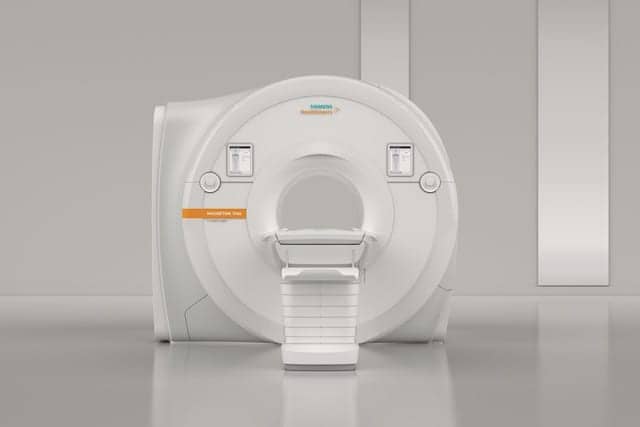
FDA has cleared the MAGNETOM Vida 3 Tesla (3T) magnetic resonance imaging (MRI) scanner from Siemens Healthineers, of Malvern, Pa. The unit features new BioMatrix technology that addresses inherent anatomical and physiological differences among patients, as well as user variability.
By reducing this variability among patients and users, the scanner’s BioMatrix technology can lower the number of rescans and increase productivity to improve MRI’s cost efficiency. BioMatrix Sensors built into the scanner’s new patient table automatically track respiratory patterns as soon as the patient lies on the table, providing information that can help formulate the optimal exam strategy.
In addition to helping users avoid costly rescans, BioMatrix Tuners improve the quality and reproducibility of whole-spine diffusion imaging via individual slice adjustments that mitigate distortion that otherwise may occur, especially at 3T. Biomatrix Interfaces help ensure consistently high exam quality, accelerating scanning by up to 30% and improving patient care. The scanner’s intuitive user interface permits correct one-touch positioning of the patient table based on intelligent body models. The patient table provides motorized assistance, enabling users to move patients who can be difficult to move (i.e. immobile, trauma, and extremely heavy patients) to and from the scanner.
The new system architecture of the MAGNETOM Vida also offers high performance and long-term stability without the need for additional space. The scanner’s 60/200 XT gradient system offers the most powerful commercially available gradients in a 70-cm bore scanner. The 55x55x50 cm field of view enables coverage of larger body regions in one step.
The GO technologies of the MAGNETOM Vida automate and simplify workflows from the start of the scan through quality control of the image data. The result is increased productivity for routine examinations of the brain, spine, and joints – from touch-of-a-button patient positioning to the transfer of clinical images to the picture archiving and communication system. The system’s new user interface not only enables automated image acquisition and processing, but also allows advanced post-processing applications to run at the scanner.
The MAGNETOM Vida also includes the company’s Compressed Sensing Cardiac Cine clinical application, which can accelerate MRI scans by a factor of 10 to allow free-breathing cardiac MRI examinations.
“With the MAGNETOM Vida, Siemens Healthineers proudly offers customers a 3T MRI system that provides unprecedented levels of personalization throughout the patient examination, to effectively address the unique challenges of each patient group and provide high-quality imaging to previously underserved segments of the population,” says Murat Gungor, vice president of magnetic resonance at Siemens Healthineers North America.