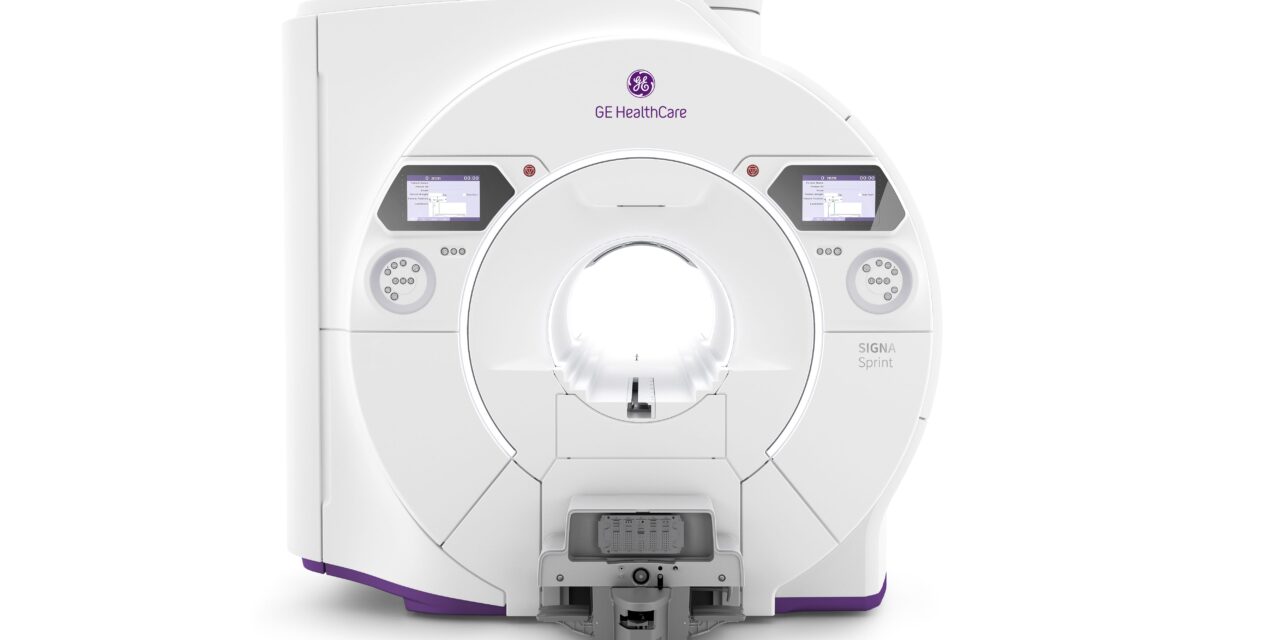
The FDA-pending wide bore 1.5T MRI scanner is designed to provide power previously unattainable at 1.5T for clarity in cardiology and oncology imaging.
GE HealthCare today unveiled SIGNA Sprint, a US Food and Drug Administration 510(k) pending wide bore 1.5T high-performance gradient MRI system, at the International Society for Magnetic Resonance in Medicine (ISMRM) 2025. This technology is designed to advance imaging possibilities in cardiology, oncology, and other clinical and research areas.
According to a release from GE HealthCare, the need for advanced diagnostics in cardiology and oncology continues to grow, with cardiovascular disease accounting for 32% of global deaths, and cancer accounting for nearly 10 million deaths per year. “Meeting this demand with advanced MRI technologies has the potential to make significant impact for cardiology and oncology patients,” says the company in the release.
With the goal of delivering higher gradient benefits previously attainable on only 3.0T systems, SIGNA Sprint is designed to offer high-performance scanning made simple at 1.5T, with “exceptional diffusion imaging,” a critical tool in oncology diagnosis and treatment planning. SIGNA Sprint is also designed to equip clinicians and researchers with the ability to expand the current 1.5T boundaries, aiming to enable fast clinical translation and pioneering research.
SIGNA Sprint, with a high-gradient performance of 65/200 per axis for fast imaging and image quality, is designed to deliver clear visualization of sub-millimetric structures and deep learning solutions to support diagnostics and treatment response monitoring in oncology patients. This new system supports clinicians’ shift to quantitative MRI and a deeper understanding of tissue characteristics, going beyond basic assessment of anatomy. The adoption of deep-learning reconstruction techniques for accelerated cardiac MRI aims to reduce the time and expertise needed to interpret scans and drive consistency and reliability.
“We are driven to push the boundaries of what’s possible in MRI with our ultra-premium segment, as our goal is to set a new standard in diagnostic research and precision care that allows for earlier clinical detection and treatment response,” says Kelly Londy, CEO, MR, GE HealthCare, in a release. “We are working to enhance diagnostic capabilities to provide highly accurate imaging with peace of mind for patients. We hope to help clinicians unlock new horizons for research in advanced imaging.”
SIGNA Sprint also is designed to optimize patient comfort, providing a 70cm wide bore space, free-breathing capabilities, and blanket-like AIR Coils to improve the patient experience. This platform is designed to benefit from built-in AI technologies: AIR Recon DL, Sonic DL, and AIR x. SIGNA Sprint aims to support clinician goals of providing precision care through the entire care pathway, from the early diagnostic stage to treatment monitoring.
SIGNA Sprint is 510(k) pending at the US Food and Drug Administration and is not yet CE marked. The system is not available for sale in any region.
Latest in MRI Advancements to be Showcased at ISMRM
GE HealthCare also will showcase the latest advancements in MRI technologies at ISMRM, including:
- SIGNA MAGNUS: A head-only MR scanner designed to support high resolution, high signal-to-noise ratio, diffusion-weighted imaging, and short scan times. GE HealthCare has demonstrated leadership in high-performance gradient technology with the introduction of HyperG gradients, one of the most efficient gradient coils on the market.
- Sonic DL 3D: Designed to accelerate MRI scans across a wide range of clinical applications, Sonic DL for 3D builds on the success of AIR Recon DL, which has helped more than 50 million patients to date.
- Freelium: a helium-free sealed magnet platform in development aims to dramatically reduce liquid helium usage without sacrificing power efficiency, operational security, and clinical performance.
ISMRM is taking place through May 15, in Honolulu, Hawaii.
Photo caption: SIGNA Sprint
Photo credit: GE HealthCare