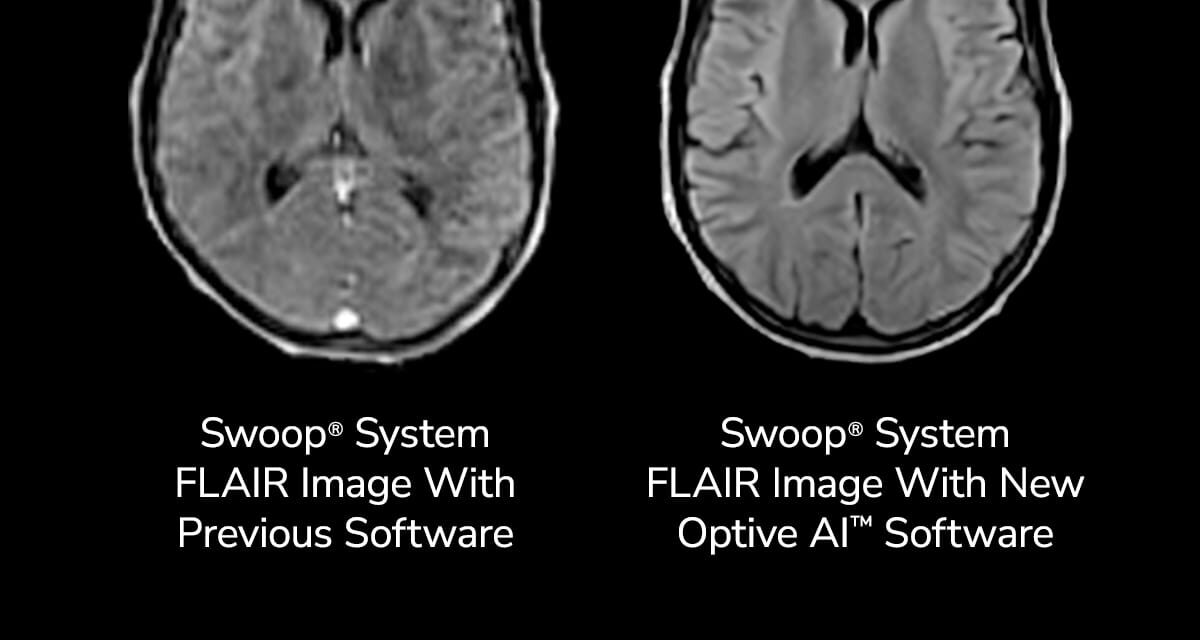
The software is designed to enhance image quality for AI-powered portable magnetic resonance brain imaging.
Hyperfine Inc—maker of a US Food and Drug Administration (FDA)-cleared AI-powered portable MRI system for the brain, the Swoop system—has received FDA clearance of its next-generation software, Optive AI. This tenth-generation release is designed to deliver improved image quality for ultra-low-field magnetic resonance (MR) imaging.
Optive AI software aims to enhance each stage of the imaging process, from noise cancellation and image acquisition to reconstruction and post-processing. The result is brain images with greater clarity, uniformity, and sharper anatomical detail, according to a release from Hyperfine. These AI algorithms are applied across all sequences.
Earlier this year, Hyperfine released the software at select clinical sites where early users responded positively to the image quality improvements, with some reporting that image quality is approaching that of conventional 1.5 tesla MRI scanners, according to the company.
“The advanced AI algorithms integrated into our new software platform dramatically elevate image quality at ultra-low field strength, enabling more confident diagnoses at the point of care…It’s the strongest signal yet of where we’re headed—and how far AI-powered portable MRI imaging can go,” says Rafael O’Halloran, Hyperfine vice president of technology, in a release.
Hyperfine plans to initiate the rollout of Optive AI software to accounts in the third quarter of 2025.
Photo caption: Comparison of Swoop system FLAIR images showing current image quality and new Optive AI software image quality.
Photo credit: Hyperfine