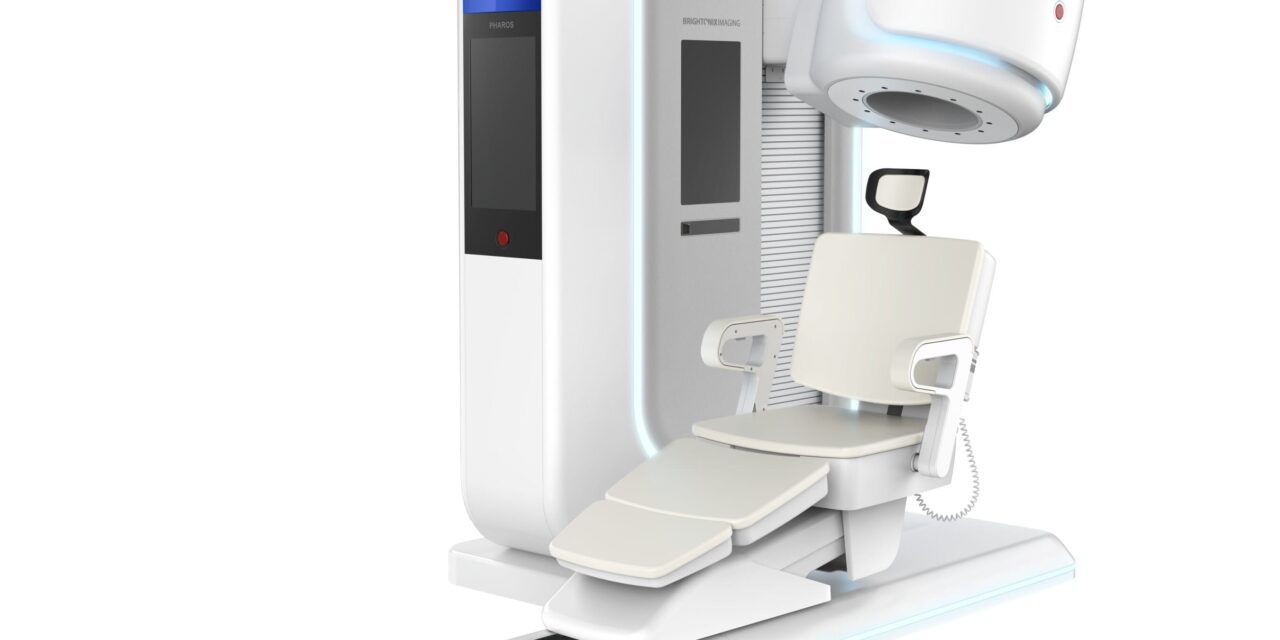
The system features a space-efficient footprint and flexible configurations for brain, breast, and extremity imaging.
Brightonix Imaging has received US Food and Drug Administration (FDA) clearance for its Pharos positron emission tomography (PET) scanner, clearing the system for commercial distribution in the US. The clinical PET system is designed with a focus on neurology applications and features a multi-functional design for various imaging needs.
According to the company, the PHAROS scanner utilizes advanced detector technology to deliver high-resolution images, aiding clinicians in early disease detection and diagnosis. A key feature of the system is its physical flexibility; the patient seat and detector configurations can be oriented for extremity and breast imaging. For brain imaging, the system can convert between both lying and seated modes.
From an installation and operational perspective, the scanner is designed with a space-efficient footprint to allow for easier integration into clinical environments where space may be limited. The company also notes that the system includes an intuitive interface intended to streamline workflow for clinicians and technologists.
“Receiving FDA clearance for the Pharos PET scanner is a monumental achievement for us and for the entire medical imaging community,” says Jae Sung Lee, CEO and founder at Brightonix Imaging, in a release. “This technology will empower healthcare providers with the tools to detect and treat neurodegenerative diseases earlier and with greater precision, ultimately improving patient outcomes.”
The development of the Pharos system was supported by the Korea Medical Device Development Fund. With FDA clearance secured, Brightonix Imaging plans to begin rolling out the scanner to medical facilities across the United States.
Photo caption: Pharos PET system
Photo credit: Brightonix Imaging