The CDC is investigating a multistate cluster of Paraburkholderia fungorum detected in patient blood cultures and linked to non-sterile ultrasound gel.
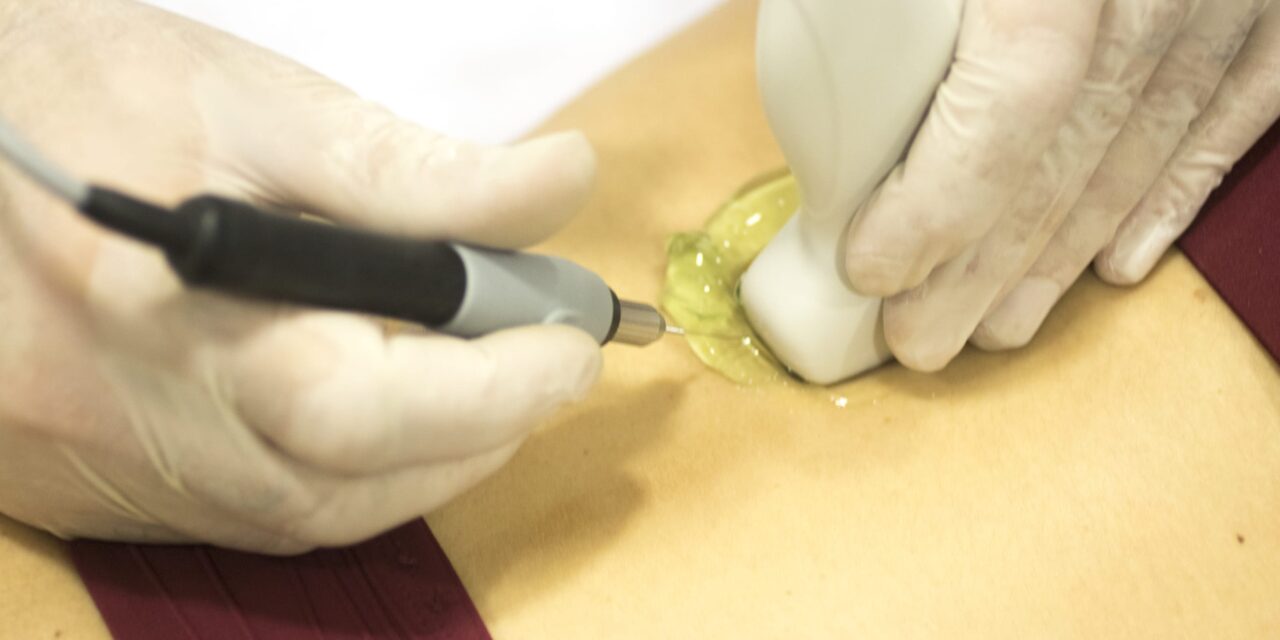
The Centers for Disease Control and Prevention (CDC) is investigating reports of an environmental bacterium linked to the use of non-sterile ultrasound gel in percutaneous procedures across multiple states between 2024 and 2025.
The bacterium, Paraburkholderia fungorum, was identified in patient blood cultures and found to be genetically similar to isolates recovered from non-sterile ultrasound gel products. The CDC has linked the organism to at least two gel brands—MediChoice and ClearImage—manufactured by NEXT Medical Products Company, with specific product lots currently under scrutiny.
Use of non-sterile ultrasound gel in percutaneous procedures—which involve puncturing the skin, such as during central line placement, amniocentesis, or biopsies—can pose a serious risk to patient safety. Even in the absence of known contamination, microorganisms in non-sterile gel can enter sterile body sites, leading to infections or false-positive cultures that may result in unnecessary treatments.
As of May 8, 2025, the CDC is aware of 40 P. fungorum isolates primarily identified in blood cultures from patients in four US states and two other countries. Some of these patients were known to have undergone ultrasound-guided percutaneous procedures before sample collection. In most cases, the patients did not appear to be clinically infected, but the presence of the bacterium still poses concerns for contamination and potential harm.
In response, the CDC recommends healthcare facilities use only single-use ultrasound gel products labeled as “sterile” for percutaneous procedures. It also advises that healthcare personnel be properly trained in distinguishing between sterile and non-sterile products, noting that terms like “bacteriostatic” or “preservative” do not equate to sterility.
ID 133876812 © Edward Olive | Dreamstime.com