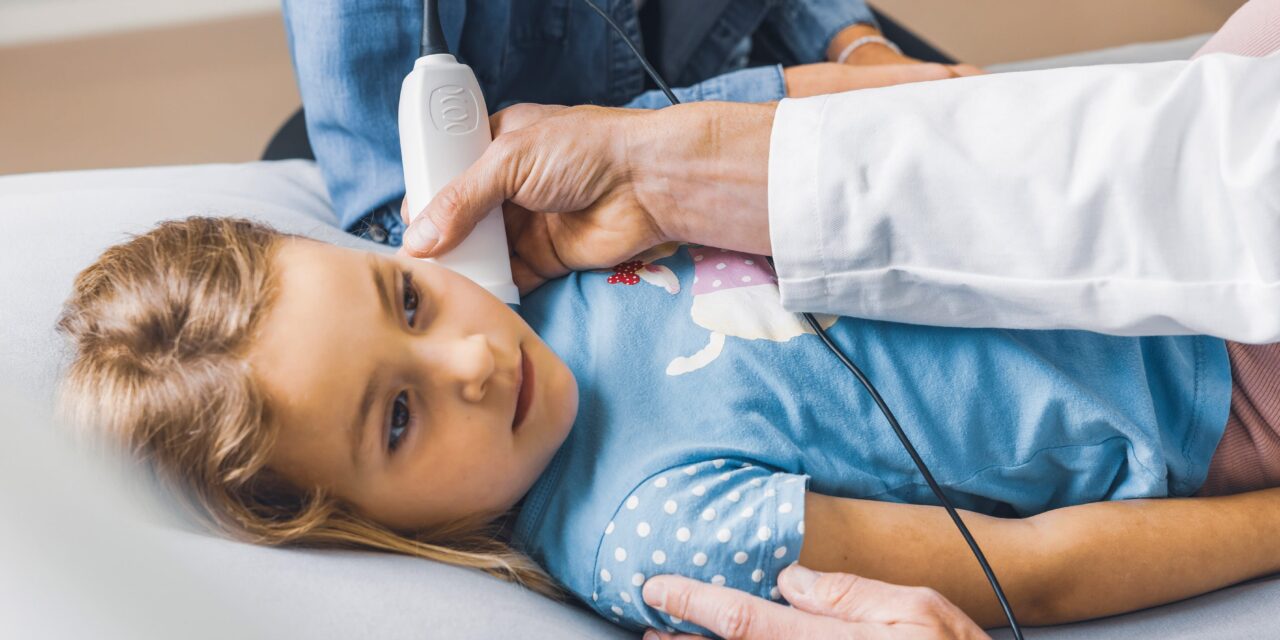
Children’s National Hospital is partnering with Compremium AG to bring new non-invasive diagnostic medical devices to the commercial market.
Summary:
Children’s National Hospital has partnered with Swiss medical device company Compremium AG to develop and commercialize non-invasive diagnostic technologies for pediatric patients. The collaboration will focus on advancing real-time, AI-driven pressure monitoring solutions, starting with central venous pressure (CVP) monitoring for congenital heart disease patients. As part of the agreement, Children’s National will serve as a Pediatric Innovation Hub, leading clinical studies and commercialization efforts to bring these technologies to market. The initiative aims to reduce reliance on invasive procedures, improve cardiovascular health monitoring, and set the stage for broader applications in pediatric and adult care.
Key Takeaways:
- Non-Invasive Pediatric Monitoring Focus – The collaboration aims to develop and commercialize non-invasive diagnostic technologies, starting with central venous pressure monitoring for congenital heart disease patients.
- AI-Driven Innovation – Compremium’s technology integrates AI to enhance real-time pressure monitoring, improve accuracy, and provide predictive insights for pediatric care.
- Children’s National as an Innovation Hub – Children’s National will lead clinical studies, research projects, and commercialization efforts to advance non-invasive diagnostic solutions for pediatric patients.
Children’s National Hospital announced a new collaboration with Swiss medical device company Compremium AG to co-develop and commercialize medical technologies designed specifically for pediatric patients.
This agreement leverages the expertise of Children’s National in pediatric healthcare innovation and Compremium’s non-invasive platform technology for diagnosing pressure-related conditions, aiming to accelerate advancements in pediatric medicine.
Diagnosing pressure-related conditions without complicated, invasive procedures has long been a challenge in the medical community. Compremium’s technology aims to improve the diagnosis of pressure physiologies, including venous pressure, intracranial pressure, and tissue pressure conditions with its non-invasive, point-of-care solution. Through this collaboration, Children’s National will serve as the Pediatric Innovation Hub for Compremium, leading clinical studies, research projects, and commercialization efforts focused on non-invasive pressure monitoring and other technologies.
“This partnership is a pivotal step in advancing pediatric-specific technologies to address critical gaps in care,” says Kolaleh Eskandanian, PhD, MBA, vice president and chief innovation officer at Children’s National, in a release. “Compremium’s non-invasive, real-time pressure monitoring technology not only has the potential to transform pediatric care but also demonstrates how prioritizing pediatric innovation can pave the way for broader adoption and scalability to adult populations.”
Initial Focus on Congenital Heart Disease Patients
The initial area of focus will be the use of Compremium’s technology in congenital heart disease patients. Pediatric cardiologists require frequent, non-invasive monitoring of central venous pressure (CVP)—a crucial measure of blood volume returning to the heart and overall circulatory function. Improved CVP data could provide new insights into cardiovascular health, potentially leading to better interventions and preventing long-term complications such as developmental delays and learning difficulties, which affect approximately 33% of congenital heart disease patients nationwide.
“Non-invasive CVP monitoring for the pediatric population is the holy grail of cardiac care, a long-awaited advancement that has the potential to revolutionize care,” says Yves d’Udekem, MD, PhD, chief of the Division of Cardiac Surgery at Children’s National, in a release. “Pediatric cardiac surgeons worldwide are eager for this breakthrough solution, which could transform how we monitor and treat cardiovascular patients. It’s exciting to see this innovation, and we are thrilled that Children’s National is leading the way in bringing it to children everywhere.”
Eskandanian adds in a release, “By integrating Compremium’s cutting-edge technology with AI-driven advancements, we aim to enhance accuracy, enable real-time data analysis, and provide predictive insights—critical capabilities for improving pediatric care. Our pediatric providers are eager for innovative, child-friendly monitoring solutions that reduce the need for invasive procedures, and this collaboration is dedicated to making that a reality.”
Advancing Regulated Medical Products
The agreement was led by Children’s National Innovation Ventures, the commercialization arm of Children’s National Hospital, specializing in advancing regulated medical products that improve pediatric health. With a focus on industry partnerships, Innovation Ventures’ four core pillars focus on advancing pediatric-driven technologies, building strategic industry alliances, facilitating funding to de-risk and validate innovations, and managing intellectual property and licensing to drive commercialization.
Additionally, Innovation Ventures leads two federally-funded accelerator programs, supporting early-stage companies and academic teams in navigating regulatory, reimbursement, clinical, and commercialization pathways.
“We are honored to collaborate with Children’s National Hospital,” says Vincent Baumann, CEO of Compremium AG, in a release. “This initiative allows us to accelerate and expand the clinical adoption of our solution, particularly for a vulnerable patient population.”
Photo caption: Children’s National Hospital has partnered with Swiss medical device company Compremium AG to develop and commercialize non-invasive diagnostic technologies for pediatric patients.
Photo credit: Children’s National