The investigational device exemption study will evaluate the safety and effectiveness of the system for hysterectomy procedures.
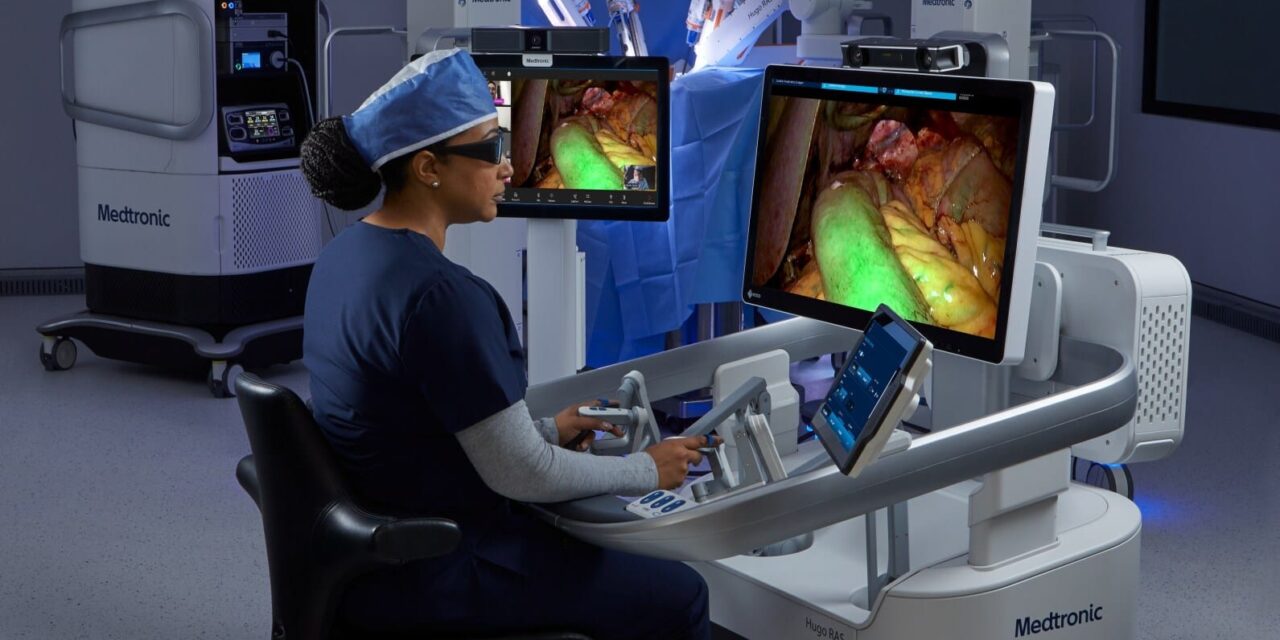
Medtronic has initiated a US clinical study for its Hugo robotic-assisted surgery (RAS) system to evaluate its safety and effectiveness in gynecological procedures. The first procedures in the study, total hysterectomies, have been completed at AHN West Penn Hospital in Pittsburgh, Pa.
The prospective multicenter study, called Embrace Gynecology, will evaluate the Hugo RAS system during radical, modified radical, or total hysterectomies, including for patients being treated for malignancies. The investigational device exemption (IDE) study plans to enroll up to 70 patients across as many as five US hospitals.
“We are excited to initiate this important clinical study, which aims to investigate surgical treatment options for women in the US,” says Emma Rossi, MD, Embrace Gynecology national principal investigator and associate professor of obstetrics and gynecology at Duke University, in a release. “In my experience, women facing a gynecologic diagnosis want two things: to effectively treat their condition and to get back to their full lives as quickly as possible. Robotic-assisted surgery helps make that possible.”
As a form of minimally invasive surgery, robotic-assisted procedures can offer patients fewer complications, shorter hospital stays, and faster return to normal activities compared to open surgery.
This is the third IDE clinical study for the Hugo RAS system in the US, following trials for urology and hernia repair. According to the company, both the Expand URO and Enable Hernia Repair studies met their primary safety and effectiveness endpoints.
Medtronic’s first US submission for the Hugo RAS system, for a urology indication, is currently under review by the Food and Drug Administration. The company plans to follow that with submissions for hernia repair and gynecology indications.
The Hugo RAS system is an investigational device in the US and is not for sale. It is commercially available in other regions, including the European Union where it is CE marked and is in clinical use in more than 30 countries.
Photo caption: Hugo console
Photo credit: Medtronic