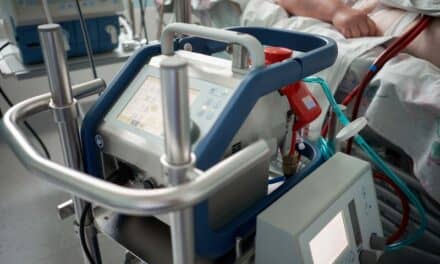
The U.S. FDA announces that it has taken significant action to help increase the availability of ventilators and accessories, as well as other respiratory devices, during the COVID-19 pandemic to support patients with respiratory failure or difficulty breathing.
“The FDA’s new actions will mean America can make more ventilators during this crisis,” says Health and Human Services Secretary Alex Azar. “With this boost from the FDA, medical device makers can more easily make changes to existing products, such as changes to suppliers or materials, to help address current manufacturing limitations or supply shortages.”
Azar adds: “Other manufacturers, such as auto makers, can more easily repurpose production lines to help increase supply. Hospitals and other healthcare providers can repurpose machines they have now to serve as ventilators. HHS and FDA’s message is clear: If you want to help expand production of ventilators to save American lives in this pandemic, we are going to work with you to sweep every possible barrier out of your way.”
The guidance issued today outlines several key steps:
First, the guidance describes the agency’s intention to exercise enforcement discretion for certain modifications to these FDA-cleared devices. Normally, any time a manufacturer or user makes a modification to a ventilator device, for instance, adding wireless and/or Bluetooth capability for remote monitoring, those modifications can often trigger an FDA premarket review, which can delay the time it takes to get these devices to the bedside.
The guidance also helps manufacturers ramp up their manufacturing by adding production lines or alternative sites, for instance, using non-medical device manufacturers such as automobile manufacturers, to start manufacturing ventilator parts. In recognition of the current pandemic situation, and to ease regulatory burden on manufacturers, the FDA is being flexible in not enforcing the premarket review requirement for these modifications.
Second, as outlined in the guidance, hospitals and healthcare professionals may use ventilators intended for other environments. For example, the guidance notes hospitals that could repurpose ventilators normally used for transporting patients in an ambulance into the hospital setting for long-term use. The FDA also provides recommendations for other alternatives that should be considered such as devices for treating sleep apnea, continuous positive airway pressure devices. The FDA’s policy also applies to healthcare facilities that use ventilators beyond their indicated shelf life, which should increase ventilator capacity.
Finally, the agency encourages manufacturers, whether foreign or domestic, to talk to the FDA about pursuing an emergency use authorization (EUA), which would allow them to distribute their ventilators in the United States. This includes U.S.-based manufacturers that were previously engaged in making medical devices, but which have capabilities to increase supply of these devices.
The FDA has been working closely with personal protective equipment (PPE) manufacturers to understand their supply capabilities during this pandemic. The agency is also aware of challenges throughout the supply chain that are presently impacting the availability of PPE products and is taking steps to mitigate shortages that healthcare facilities are already experiencing.
For example, on March 2, the FDA granted an Emergency Use Authorization to allow National Institute for Occupational Safety and Health-approved respirators typically used in industrial settings to be used in healthcare settings. The agency has also published a Letter to Healthcare Providers and FAQs that provide conservation strategies for gowns and masks and continues to coordinate and communicate with interagency and state partners to help ensure that they are more readily available.
Late last week, the agency also issued a Letter to Health Care Providers sharing conservation strategies for surgical gloves, recognizing the need for PPE, such as medical gloves, may outpace the supply available to healthcare organizations during the COVID-19 pandemic. Proposed conservation strategies include using nonsterile disposable patient examination gloves for routine patient care or using medical gloves beyond the manufacturer-designated shelf life in a setting where there is a lower risk of transmission.