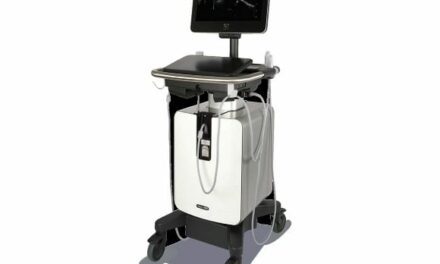
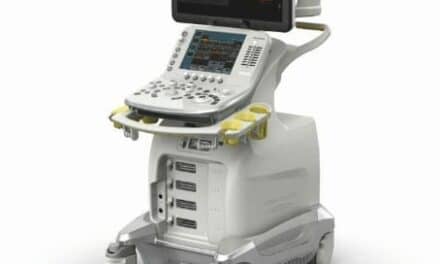
The $35 million expansion will advance artificial intelligence-powered ultrasound solutions for trauma assessment and mass casualty incident preparedness.
GE HealthCare announced an approximately $35 million expansion to its existing contract with the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the US Department of Health and Human Services.
The cost-share agreement, with BARDA providing the majority of funding, builds on a program launched in October 2023 to develop artificial intelligence (AI)-powered ultrasound solutions for trauma assessment and mass casualty incident preparedness.
“Responding to a mass casualty incident demands speed, precision, and access to care, especially in high-pressure environments like emergency departments, field hospitals, and medical transport,” says Philip Rackliffe, president and CEO of advanced visualization solutions at GE HealthCare, in a release. “Our strategic alignment with BARDA enables us to continue to drive ultrasound innovation through advanced AI tools and specialized hardware designed for the front lines of care.”
New AI Tools for Emergency Care
The expanded program will facilitate development of several new AI-powered tools designed to enhance diagnostic speed and reduce operator dependency for non-expert ultrasound users. The automated capabilities will support more detailed assessments of lung pathologies, including pleural pathologies, and improve detection of intra-abdominal injuries.
GE HealthCare will also create point-of-care ultrasound solutions designed to improve reliability and usability in demanding environments, including field-based care settings.
“GE HealthCare has long been at the forefront of ultrasound innovation in emergency medicine, especially at the point-of-care,” says Karley Yoder, CEO of comprehensive care ultrasound, advanced visualization solutions at GE HealthCare, in a release. “This milestone reflects our shared aspiration to equip clinicians with tools that enhance decision-making and help improve patient outcomes in even the most challenging scenarios.”
Clinical Validation and Evidence Generation
Beyond technology development, the expanded program enables new efforts to engage with clinicians and medical evaluation sites. These activities will help generate clinical evidence, inform ongoing development, and ensure the technology is shaped by real-world feedback.
By working directly with experts in emergency medicine, GE HealthCare aims to validate the impact of these innovations and support broader adoption across diverse healthcare environments.
The project is supported with federal funds from the US Department of Health and Human Services; Administration for Strategic Preparedness and Response; BARDA under contract number 75A50123C00035.
ID 42488480 © Wayne Mckown | Dreamstime.com