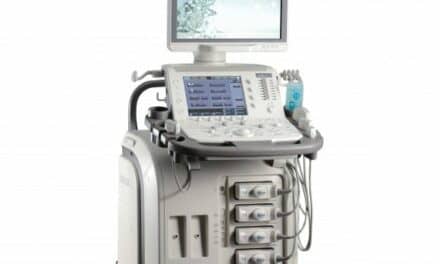
Fujifilm SonoSite subsidiary Fujifilm VisualSonics has received FDA 510(k) clearance for its Vevo MD ultra-high-frequency ultrasound system. Designed for vascular, musculoskeletal, dermatological, and neonatological applications, the Vevo MD enables clinicians to visualize anatomy within the first 3 cm of the body.
Renaud Maloberti, vice president and general manager of Fujifilm VisualSonics, says the ultrasound system allows healthcare professionals to “see what they have never seen before: unparalleled image resolution down to 30 micrometers.” For reference, he says, this is more than half the size of a grain of sand. “Imagine the great potential this technology has across unexplored applications to visualize the smallest, highly detailed anatomy in a way that has never been done before,” Maloberti adds.
The Vevo MD, which received the European CE mark in January, is compatible with Fujifilm VisualSonics UHF transducers. Such compatibility has strong implications for the ultrasound system, company officials say, since the transducer technology is capable of operating at a frequency of up to 70 MHz—higher than most ultrasound systems.
Masayuki Higuchi, Fujifilm SonoSite’s president and CEO, calls the FDA clearance a major step in getting this technology to market. “This powerful and innovative technology offers unprecedented image resolution capabilities that will have significant impact on the US medical imaging community and the care providers deliver to patients,” he adds.
For more information about this system, visit Fujifilm VisualSonics.