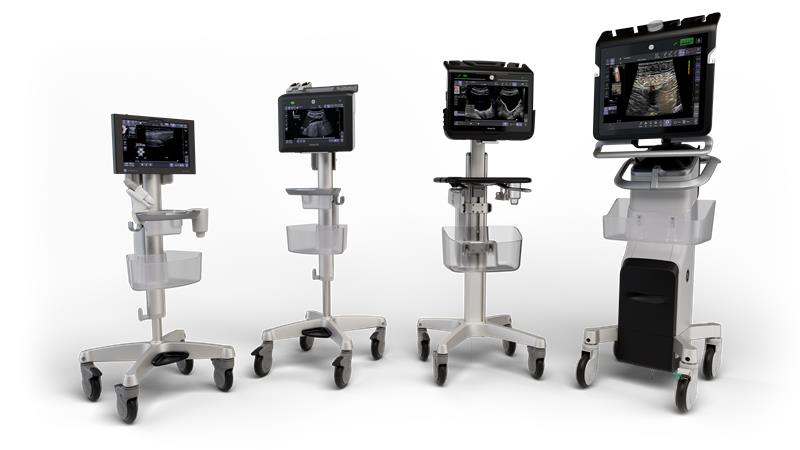
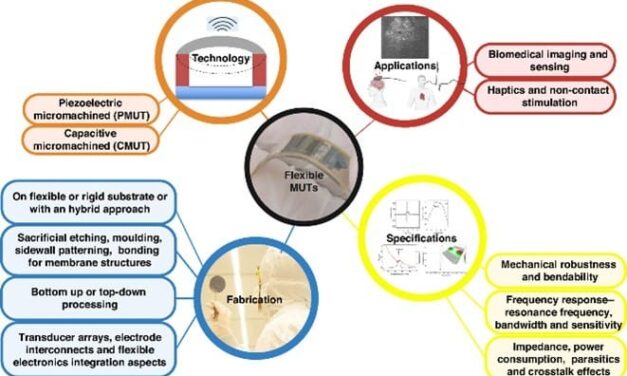
GE HealthCare, BARDA Expand Collaboration on AI Ultrasound for Emergency and Mass Casualty Response
The $35 million expansion will advance artificial intelligence-powered ultrasound solutions for trauma assessment and mass casualty incident preparedness.