The agency reminds healthcare facilities to follow manufacturer instructions for use and maintenance after reports of fires, serious injuries, and deaths.
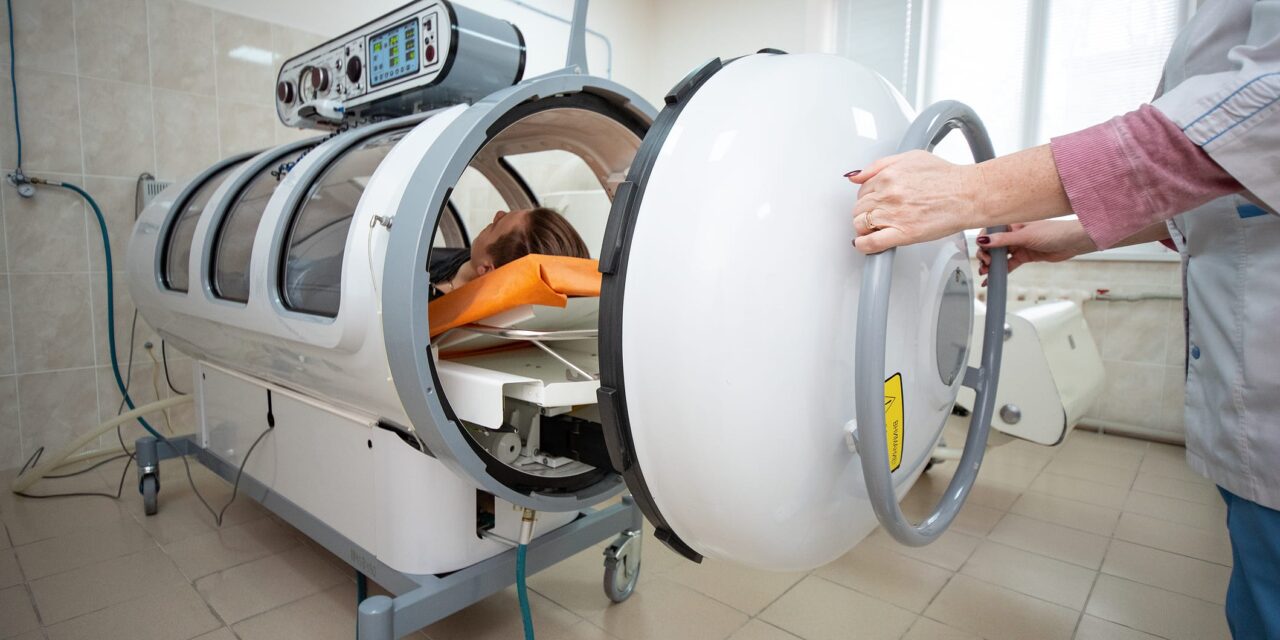
The US Food and Drug Administration (FDA) has issued a letter to healthcare providers reminding them of the importance of safe use protocols for hyperbaric oxygen therapy (HBOT) devices. The communication comes after the agency received reports of serious injuries and deaths with the use of HBOT.
While the FDA notes that the root cause of these events is not yet known and believes serious adverse events are rare, it is urging facilities to follow manufacturer instructions to reduce potential risks. HBOT involves breathing 100% oxygen in a pressurized chamber. The devices are regulated as Class II medical devices.
The FDA highlighted several key recommendations. These include ensuring that manufacturer-recommended cleaning procedures, maintenance intervals, and safety checks are strictly followed for each HBOT device. The agency also stressed the importance of using proper grounding equipment and providing and maintaining training for all staff who operate the devices.
The letter specifically points to the heightened risk of fire associated with using oxygen at high concentrations. It advises facilities to ensure all fire prevention measures are followed and to adhere to manufacturer instructions on prohibited items, such as certain electrical or static-generating devices. The FDA also noted that patient clothing made of materials like wool or synthetics can produce more static electricity than recommended materials such as cotton.
The FDA stated it will continue to work with manufacturers and professional societies to encourage training and safe use of HBOT devices. The agency is encouraging healthcare providers to report any adverse events through MedWatch, its Safety Information and Adverse Event Reporting program, to help better understand the risks associated with these devices.
ID 142345327 © Svitlana Nakleishchykova | Dreamstime.com