GE HealthCare Introduces CareIntellect for Perinatal to Streamline Maternal and Fetal Monitoring
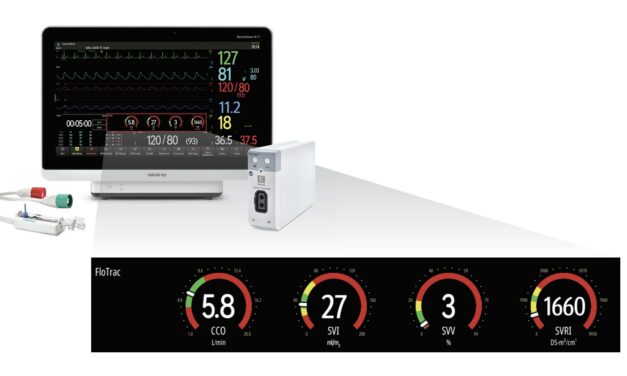
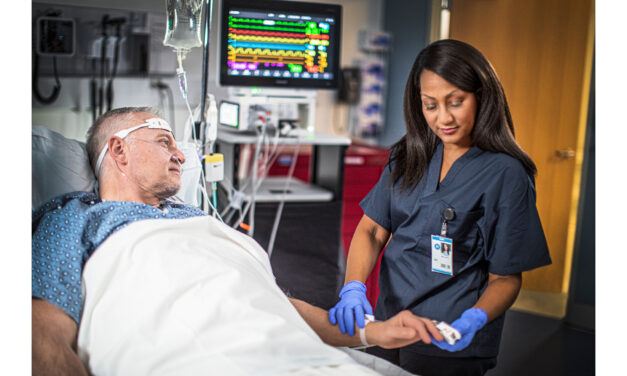
The cloud-based solution integrates maternal and fetal vital signs—such as uterine activity, blood pressure, and fetal heart rate—into a unified, chronological view to support efficient decision-making.