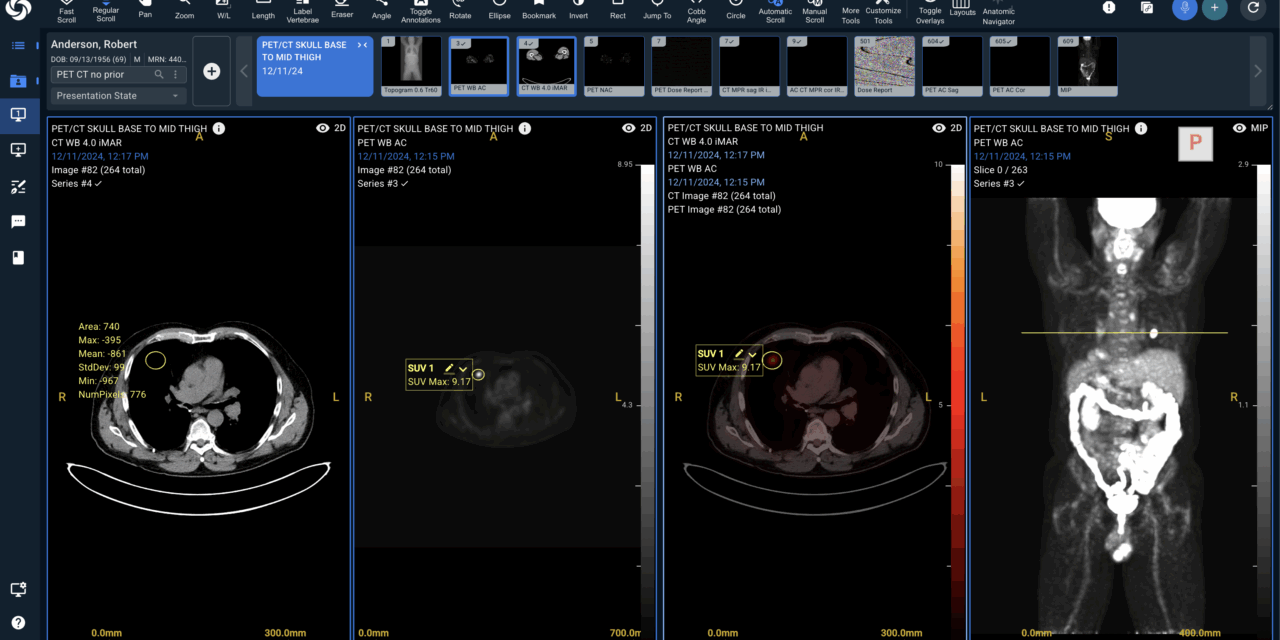
The platform’s 510(k) clearance adds PET-CT workflows—including SUV quantification, fusion, MIP, and MPR—to its browser-based diagnostic environment, advancing unified cloud imaging infrastructure.
Sirona Medical has received US Food and Drug Administration (FDA) 510(k) clearance for its Sirona Advanced Imaging Suite, expanding diagnostic imaging capabilities to include PET-CT support with quantitative SUV analysis, image fusion, maximum intensity projection generation, and multi-planar reconstruction.
“This milestone reflects our commitment to closing the most critical capability gaps that limit the flexibility and reach of modern radiology,” says Dr Peter Sachs, radiologist, imaging informaticist, and Sirona advisor, in a release. “By pioneering the field of 100% cloud-native PET viewing and PET-CT fusion, we’re enabling radiologists to interpret advanced imaging studies from anywhere—without compromise in diagnostic confidence, speed, or quality.”
The newly cleared functionality represents a step to unify the radiology workflow by bringing advanced visualization, reporting, and collaboration into a single, cloud-native environment accessible through a browser.
“With PET-CT now cleared, Sirona has completed a four-year journey toward delivering the most complete, FDA-cleared, and cloud native diagnostic environment in the industry,” says Ken Kaufman, chief executive officer of Sirona Medical, in a release. “This is a foundational milestone for our customers.”
Sirona’s Advanced Imaging Suite will begin rolling out to users, with immediate availability for existing customers participating in early-access programs.
Sirona recently hosted a webinar, “The Cloud-Native Path to Profitable Radiology,” discussing how cloud-native architecture is changing radiology practices.
Photo caption: Sirona Advanced Imaging Suite
Photo credit: Sirona Medical