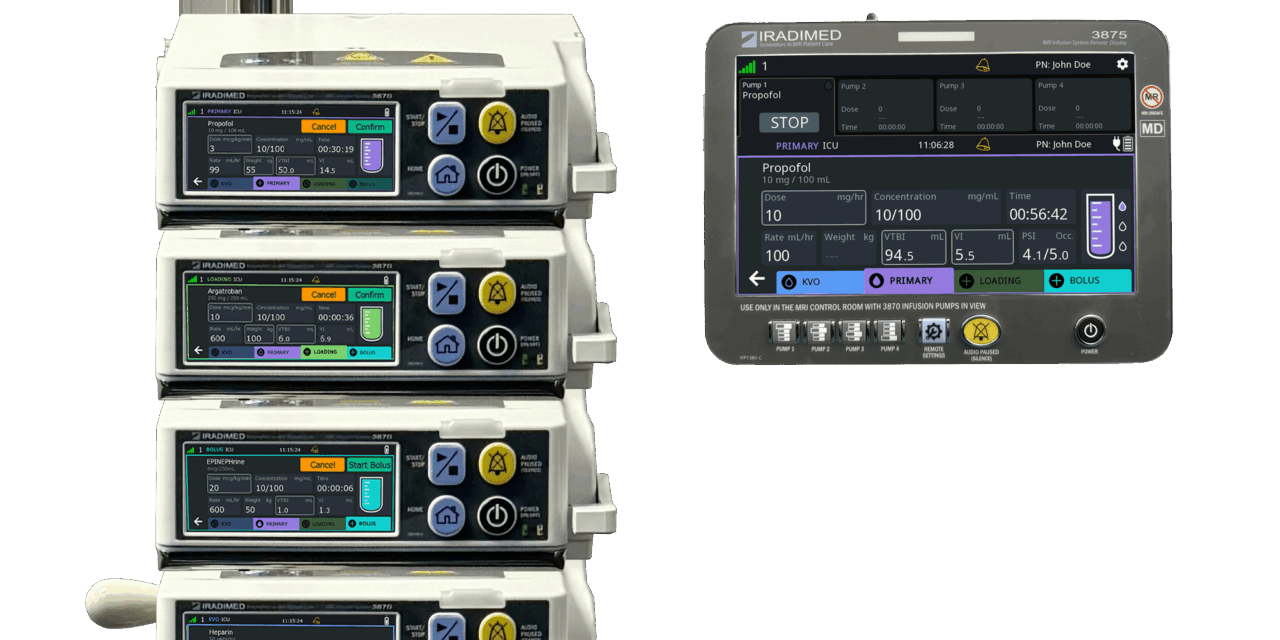
The clearance paves the way for the company to begin deployment of its next-generation MRI-safe IV pump in late 2025.
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Iradimed Corporation’s MRidium 3870 infusion pump system, the company’s next-generation magnetic resonance imaging (MRI)-compatible intravenous infusion pump.
Designed for use in MRI environments, the MRidium 3870 builds on Iradimed’s earlier systems, which the company says remain the only non-magnetic infusion pumps cleared for use during MRI procedures.
“We are thrilled to receive FDA 510(k) clearance for the MRidium 3870, a milestone that underscores our commitment to advancing MRI-compatible medical technology,” says Roger Susi, president and CEO of Iradimed Corporation, in a release. “This long-awaited clearance reflects our productive collaboration with the FDA to meet evolving and stringent regulatory requirements. The MRidium 3870 empowers clinicians to deliver critical IV fluids and medications safely and predictably in MRI environments, improving patient outcomes and operational efficiency.”
Iradimed expects to begin rolling out the MRidium 3870 at select healthcare facilities in the fourth quarter of 2025, with broader commercial distribution to follow in 2026.
The company, which received FDA clearance for its first-generation infusion pump in 2005, also manufactures non-magnetic patient monitoring systems designed for use in MRI suites. According to Iradimed, the new device expands its offerings for hospitals seeking MRI-compatible solutions in anesthesiology and critical care.
Photo caption: MRidium 3870 infusion pump system
Photo credit: Iradimed Corporation