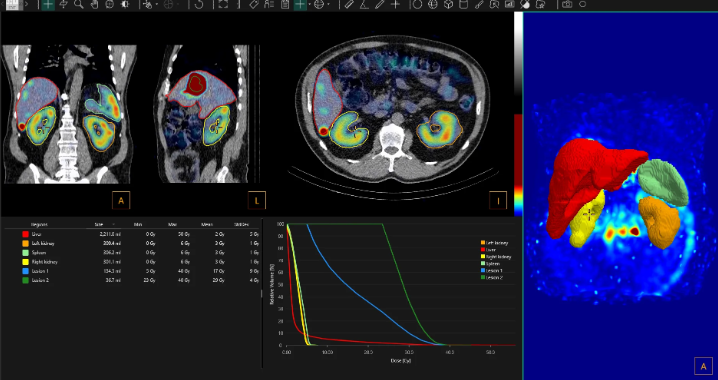
The new release adds machine learning–based organ segmentation, faster dose calculations, and automated workflow features.
Hermes Medical Solutions has received US Food and Drug Administration 510(k) clearance for the new version of Hermia Voxel Dosimetry. The product has previously been CE-marked.
Hermia Voxel Dosimetry introduces several new features intended to improve its dosimetry aspect and simplify workflows. It is furthermore optimized to calculate dose maps faster than prior versions.
“The new release of the Hermia Voxel Dosimetry is a big step forward in personalized dosimetry for theranostics. It includes additional isotopes, automated workflows, and uses the advanced Hermia Monte Carlo dose calculations. It will enable fast and easy calculations of dose received thanks to the efficient organ segmentation with machine learning algorithm,” says Tom Francke, CEO of Hermes Medical Solutions, in a release.
Some of the highlights of the latest version of Hermia Voxel Dosimetry, include:
- Semi-automatic organ segmentation (with machine learning-based segmentation algorithm)
- Improved ability to fill in information automatically from header
- Reduced calculation times
- Automated workflow configuration options
- Deformable registration for enhanced alignment, accommodating patient movement and breathing corrections
- Reduced sensitivity to noise
- Improved compatibility with Spectrum Dynamics Quantitative SPECT
- New features for research use:
- Dose rate calculations
- Add your own isotope
- Surrogate isotope calculations
Photo caption: Hermia Voxel Dosimetry
Photo credit: Hermes Medical Solutions