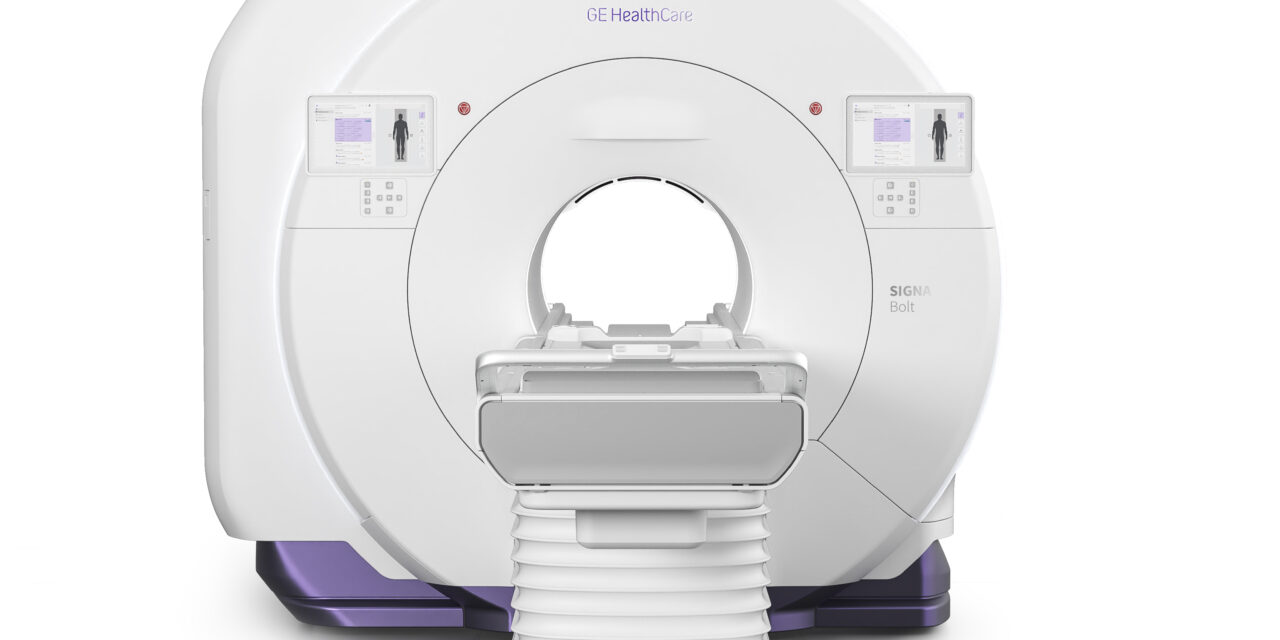
The SIGNA portfolio includes a helium-free 1.5T system, advanced 3T scanner, and AI-driven workflow platform designed to improve imaging efficiency.
GE HealthCare announced 510(k) clearance from the US Food and Drug Administration for three new magnetic resonance innovations: SIGNA Sprint with Freelium, a 1.5T sealed magnet MRI system; SIGNA Bolt, an advanced 3T MRI scanner; and SIGNA One, an AI-driven ecosystem of workflow solutions.
GE HealthCare says clearances represent a milestone for its MRI portfolio as healthcare facilities face rising demand for imaging services while managing operational constraints including helium shortages and staffing challenges.
“Achieving FDA clearance of our next-generation SIGNA MRI technology underscores our commitment to expanding access to high-quality imaging and elevating the standard of care for patients everywhere,” says Kelly Londy, president and CEO of MR at GE HealthCare, in a release. “As MRI demand continues to rise across clinical areas, providers need solutions that deliver greater efficiency without compromising diagnostic precision.”
Helium-Free Technology Addresses Supply Concerns
The SIGNA Sprint with Freelium is designed to address ongoing helium supply challenges with sealed magnet technology that uses less than 1% helium. The system maintains image quality through homogeneity and high signal-to-noise ratio while integrating deep learning solutions including AIR Recon DL and Sonic DL, according to the company.
Key operational features include a five-hour ride-through time that enables the system to withstand power outages without disruption, identical homogeneity specifications as conventional magnet technology, and the same power infrastructure requirements as current systems.
The ventless magnet design enables installation in diverse settings, from major hospitals to remote regions. The system includes two levels of operational autonomy: scanning autonomy through the SIGNA One interface and autonomous magnet monitoring that automates ramp-down and ramp-up processes without requiring field engineer support.
Premium 3T System Reduces Power Consumption
SIGNA Bolt features an advanced 80/200 gradient system that delivers research-level performance with approximately 30% lower power consumption compared to previous-generation systems. The scanner includes a new AIR Coil suite designed to enhance patient comfort and operational flexibility, including feet-first capabilities to support claustrophobic patients and reduce rescans.
The system’s infrastructure profile offers up to 65% reduction in peak power demand and up to 34% reduction in minimum equipment room space requirements.
AI Platform Streamlines Workflow
The new MRI systems are powered by SIGNA One, an AI-enabled workflow ecosystem that addresses inefficiencies across MRI operations. The platform integrates multiple solutions including a user interface designed to reduce training time, automated patient positioning with AI-enabled anatomical landmark localization, and contactless respiratory and peripheral gating.
“As imaging volumes continue to climb, we’re under constant pressure to deliver answers faster without compromising diagnostic quality,” says Tiron CM Pechet, MD, assistant medical director and CMIO at Shields Health, in a release. “What sets this new SIGNA MR platform apart is the focus on real-world clinical workflow. From the AI-driven automation to the dramatically streamlined setup and scanning process, we’re seeing meaningful reductions in exam time and far greater consistency across technologists.”
The platform includes high-resolution in-room consoles that enable technologists to control patient setup while minimizing interruptions and a fully automated camera system with real-time visual guidance on touchscreen displays.
GE HealthCare anticipates CE mark approval for the systems before the end of 2026.
Photo caption: SIGNA Bolt
Photo. credit: GE HealthCare