The newly cleared system is designed to support breast biopsy procedures across multiple imaging modalities using a single platform.
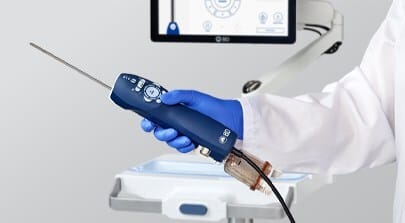
BD announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the EnCor EnCompass Breast Biopsy and Tissue Removal System, a multi-modality breast biopsy system designed to provide clinicians with flexibility across breast imaging modalities in the diagnosis of breast disease.
The EnCor EnCompass Biopsy System is designed to streamline the breast biopsy experience by enabling clinicians to perform procedures across a range of breast imaging platforms using one integrated system. The system, which is expected to enter the market in early 2026, offers a combination of advanced features and user-focused design to support procedural efficiency, according to a release from BD.
“The FDA clearance of the EnCor EnCompass Biopsy System demonstrates our ongoing focus on addressing the evolving needs of clinicians and patients in breast health,” says Stacie Watson, vice president and general manager of the oncology platform at BD Interventional–Peripheral Intervention, in a release. “This multi-modality platform is engineered to provide flexibility, control, and ease of use, with features designed to support both clinician confidence and the patient experience.”
According to the company, features of the EnCor EnCompass Biopsy System include:
- Multi-modality use to support procedures performed across breast imaging platforms
- High and low vacuum strengths and a variable sample notch that can be adjusted during the procedure
- 360° sampling capability to access lesions located throughout the breast
- Features to enhance visualization, including an echogenic cutting cannula and illuminated sample container
- Choice of 12G, 10G, and 7G probes to accommodate different lesion types and locations
“Our goal is always to provide the best possible care for patients while maintaining efficiency, accuracy, and safety,” says Shadi Aminololama-Shakeri, MD, chief of breast radiology at UC Davis, in a release. “The EnCor EnCompass Biopsy System combines multi-modality capability and enhanced control into one platform that supports intraprocedural customization and helps streamline the biopsy process.”
The clearance of the EnCor EnCompass Biopsy System expands BD’s breast health portfolio.
Photo caption: EnCor EnCompass Breast Biopsy and Tissue Removal System
Photo credit: BD