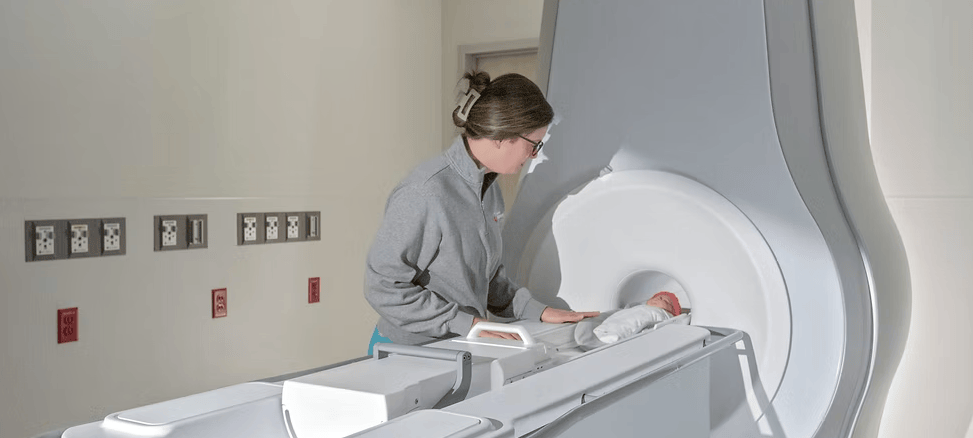
The system features a compact footprint and is designed for whole-body imaging in neonatal care settings.
Eyas Medical Imaging Inc has received US Food and Drug Administration (FDA) 510(k) clearance for its Ascent neonatal magnetic resonance imaging (MRI) system, a whole-body magnetic resonance scanner designed and optimized for neonate and infant anatomy, including head, body, and extremities.
“510(k) clearance marks a significant milestone for the company and reflects our mission to enable healthcare professionals to save more babies’ lives with state-of-the-art, precision imaging,” says Matt Storer, president and CEO of Eyas Medical Imaging, in a release.
According to a release from Eyas Medical Imaging, Ascent is the first high-field, 3 Tesla (3T) dedicated neonatal MRI system. It utilizes a 3T magnet, enabling a more comprehensive and precise diagnostic tool that provides healthcare professionals with imaging of vital anatomy, including the brain, lungs, heart, and abdomen.
Addressing Limitations of Using Adult MRI for Babies
The Ascent aims to address the technical limitations of using an adult-size MRI system to image babies and to provide clinicians with an improved ability to visualize and diagnose disease in the neonatal patient population.
A barrier to using MRI for neonates has been the risk of transporting fragile newborns from the neonatal intensive care unit (NICU) to alternate MRI locations within the hospital. Eyas’ engineering delivers a whole-body 3T neonatal MRI system with a compact footprint that allows installation in the NICU. The Ascent is virtually helium-free, according to the company, and does not require a quench pipe or outside venting.
The Ascent was conceived at Cincinnati Children’s, incorporating learning from their over 1,700 infant MRI scans on prototype systems. It includes features, such as a detachable patient table that can serve as a patient transport device, providing flexibility in NICU workflow based on hospital needs and preferences. The system also leverages advanced electronics, operating software, and pulse sequences from Philips Medical Systems Nederland B.V.
“We took great care in the design of the Ascent. Our goal is to transform neonatal care by bringing an unprecedented level of MR imaging and access to the most vulnerable patients when and where they need it,” says MR physicist Charles Dumoulin, PhD, a professor of pediatrics and radiology at Cincinnati Children’s and the founder of Eyas Medical Imaging, in a release.
The company is currently scaling up operations and expects to be in commercialization in the United States later in the year. The device is not yet commercially available in other countries.
Photo caption: Ascent neonatal MRI system
Photo credit: Eyas Medical Imaging