The system is designed to streamline quality processes and simplify adherence to ISO, FDA, and EU MDR requirements for regulated industries.
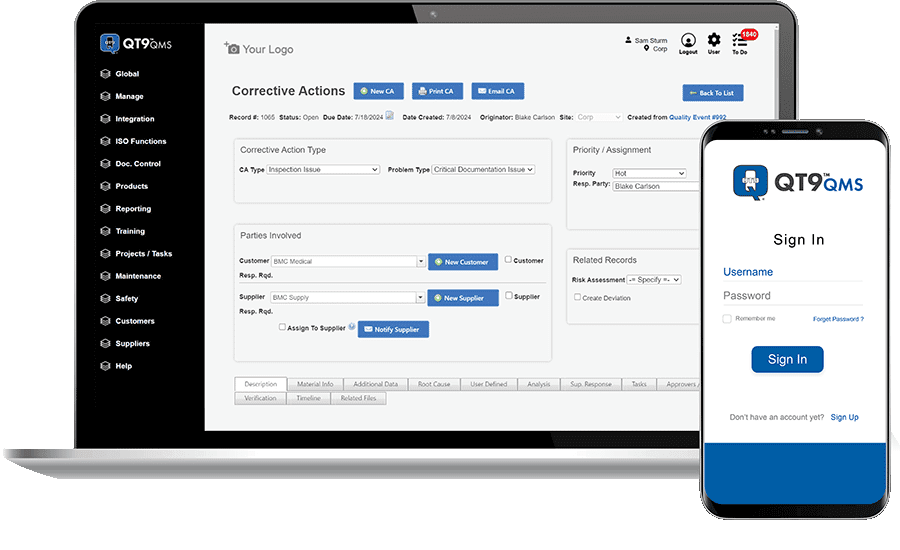
QT9 Software has announced its QT9 QMS, a quality management platform developed to assist organizations with automating quality processes and compliance workflows. The system integrates tools for managing International Organization for Standardization (ISO) and Food and Drug Administration (FDA) requirements into a single platform.
Designed for regulated environments, the system features compliance automation tools such as electronic signature approvals, automatic audit trails, revision control, and validation protocols. The platform is pre-validated for ISO, FDA, European Union Medical Device Regulation (EU MDR), and other standards to support audit readiness. It also includes built-in Installation qualification, operational qualification, and performance qualification protocols.
Operational and Quality Assurance Features
The platform’s quality assurance suite includes modules for defect tracking, incident management, and monitoring for equipment and supplier quality. It also provides centralized document control to manage structured document reviews, secure storage, revision tracking, and sharing with internal and external users.
Additional operational tools include automated corrective and preventive action management, streamlined audit scheduling, deviation monitoring, complaint and feedback tracking, inspection management, and engineering change control with electronic approvals.
System Technology and Deployment
QT9 QMS is available as a cloud-based or on-premise solution, with data residency options in the US or European Union. The system provides reporting through dashboards with filtering, sorting, and graphical presentation options. Workflow tools include to-do lists, email alerts, customizable forms, and permission controls to manage tasks and optimize processes.
The platform also offers integrated modules for team training, including assessment management, course tracking, and personalized tests. Secure web portals are available to provide dedicated access for customers, employees, and suppliers to facilitate communication and collaboration.
Photo caption: QT9 QMS
Photo credit: QT9