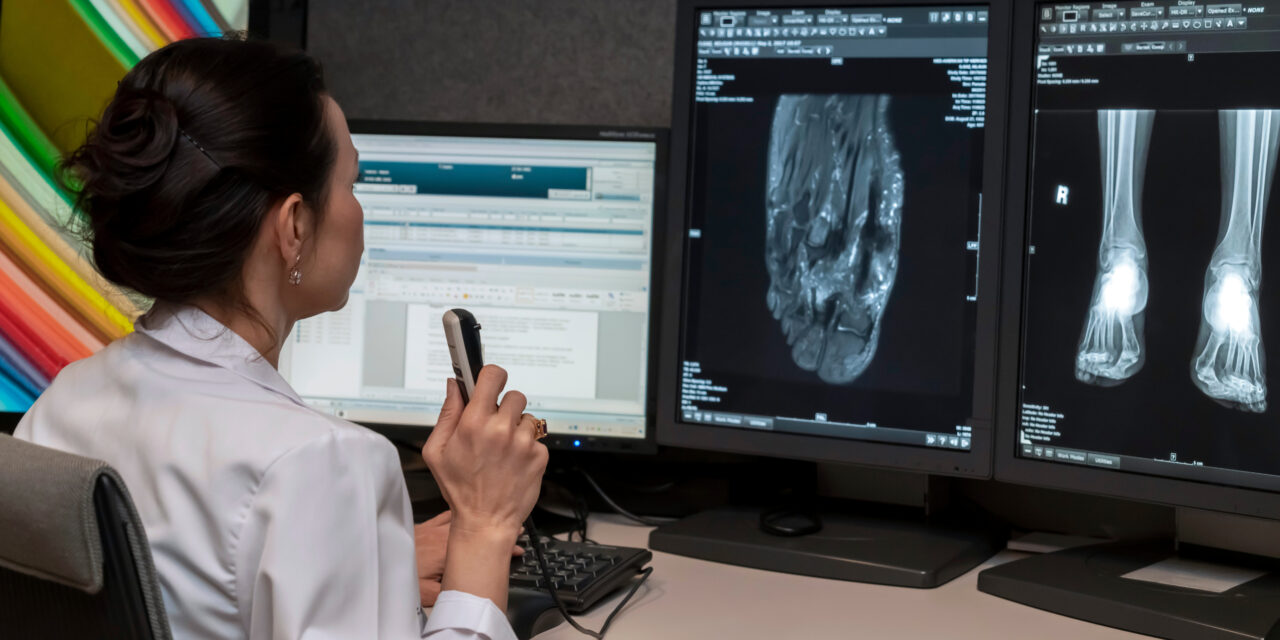
The designation will expedite the review process for the CT-based artificial intelligence, which flags numerous acute conditions simultaneously.
Aidoc has received Breakthrough Device Designation from the US Food and Drug Administration (FDA) for its multi-triage artificial intelligence (AI) solution that flags an array of time-sensitive medical conditions from computed tomography (CT) scans within a single workflow.
The FDA grants Breakthrough Device Designation to technologies that may provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases and represent an unmet clinical need. The designation is intended to accelerate the review process. For this solution, it enables the parallel review of numerous indications within a single submission, a notable step for bringing multi-use clinical AI into routine care.
Built on the company’s Clinical AI Reasoning Engine (CARE) foundation model and deployed through its aiOS platform, the solution is designed to help clinical teams identify and prioritize high-risk cases more quickly. According to the company, the technology aims to address workflow bottlenecks in emergency departments and radiology that result from rising patient volumes and imaging demand.
“With this designation and our ecosystem of specialized partners, we can deliver a broader set of solutions across many service lines. CARE’s ability to detect, characterize, measure, and compare findings underpins both current and in-development applications from triage to report drafting,” says Elad Walach, co-founder and CEO, Aidoc, in a release. “We are tackling this from multiple angles, developing innovations in parallel and, as each is FDA cleared, bringing it to market—delivering valuable, safe solutions while building toward the next milestone.”
Michael Braginsky, co-founder and CTO of Aidoc, adds in a release, “The technology creates an extraordinary opportunity to advance healthcare, but success depends on more than the AI itself. It’s about how it integrates, what data it can access, how it interacts with users, and how change is managed. It’s a massive lift, but by leveraging the expertise and infrastructure we’ve built over the years, we’re turning this vision into reality.”
Aidoc reports that its roadmap includes additional capabilities powered by CARE, such as the auto-creation of draft reports. The company currently holds 18 FDA clearances, and its technology is deployed in more than 1,600 hospitals worldwide.
Photo caption: Multi-triage artificial intelligence solution
Photo credit: Aidoc