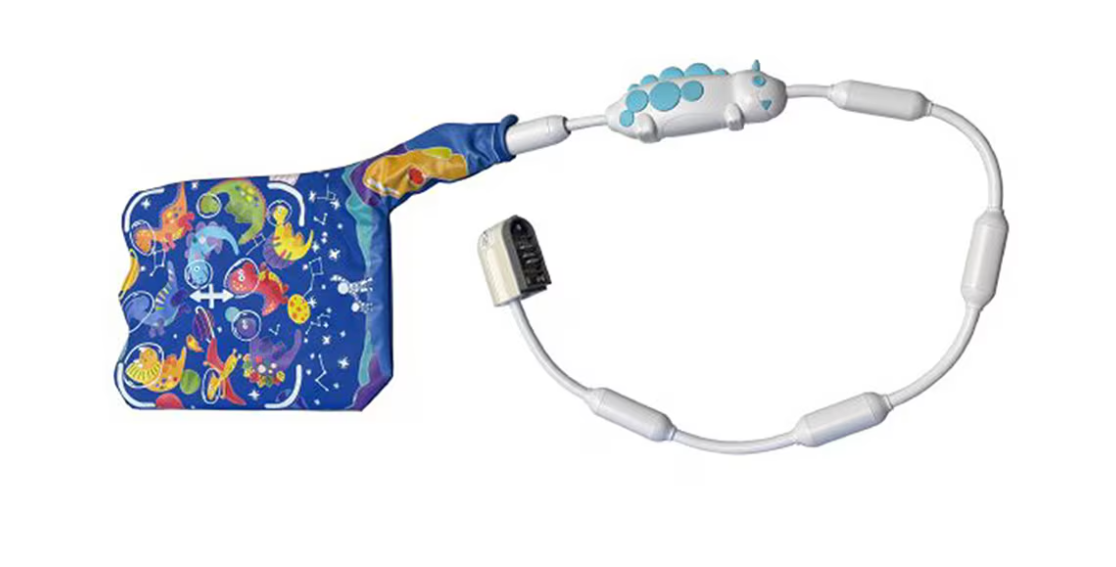
The pediatric body array coil, developed to offer a more flexible alternative to rigid devices, is optimized and validated for use on 3.0T MRI systems.
Royal Philips announced the availability of the InkSpace Imaging Snuggle pediatric body array coil for Philips 3.0T MRI systems. Designed for pediatric patients, the Snuggle coil has been optimized and validated for use with Philips 3.0T MRI systems.
Young patients often find MRI exams challenging, and the Snuggle coil was created to make the experience more comfortable and less intimidating. The Snuggle coil’s high-density array and flexible design allow high-resolution images and more efficient exams across a range of pediatric anatomies, according to a release from Royal Philips, which can reduce the need for additional imaging or follow-up procedures.
“The Snuggle coil shows how thoughtful design can make MRI scans less stressful for children while giving clinicians the image quality and workflow efficiency they rely on,” says Ioannis Panagiotelis, PhD, business leader, MR at Philips, in a release.
Recently cleared through the US Food and Drug Administration (FDA) 510(k) process, InkSpace Imaging developed the Snuggle coil to provide a more child-friendly alternative to traditional rigid devices.
“Snuggle was designed and built by InkSpace Imaging to solve a very specific pediatric challenge: comfort and performance without tradeoffs,” says Peter Fischer, CEO, InkSpace Imaging, in a release. “By enabling compatibility with Philips 3.0T MRI systems, we can bring that child-centered design to more hospitals and help care teams get the scan right the first time.”
The Snuggle pediatric coil for Philips 3.0T MR systems is now available in the United States, with rollout to additional regions planned in the future.