The device combines ultrasound elastography with AI features to help clinicians assess liver fat and stiffness at the point of care for patients with chronic liver disease.
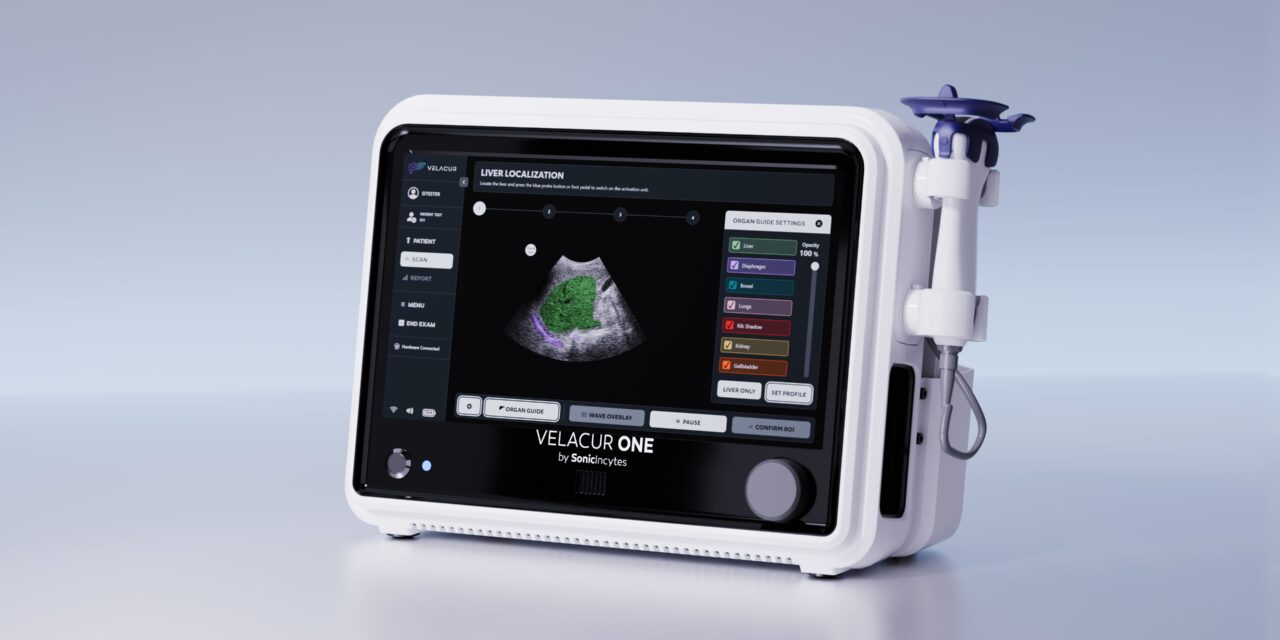
Sonic Incytes Medical Corp announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for Velacur ONE, its point-of-care ultrasound elastography device.
Building on its original model, Velacur, Velacur ONE introduces an enhanced interface and features for improved portability and user experience. These improvements are designed to enable broader scalability to support Sonic Incytes’ accelerated US and global expansion strategy.
Velacur ONE measures attenuation, Velacur determined-fat fraction, and liver stiffness using 3D S-WAVE and aids in the management of chronic liver disease including metabolic dysfunction–associated steatohepatitis (MASH) and metabolic dysfunction–associated steatotic liver disease (MASLD).
With the first therapeutic for MASH,a progressive and often underdiagnosed liver disease, now available—following the recent FDA clearance of Rezdiffra—clinicians need improved non-invasive tests to diagnose and monitor MASH at point-of-care.6 Current treatment guidelines for MASH recommend imaging-based elastography, such as Velacur, to assess liver scarring and fat content. While ultrasound elastography is widely used, when it comes to treating patients with MASH, liver stiffness alone cannot be relied on to assess treatment response in the short term.7 The best predictor of treatment responses was a decrease in steatosis (identified as ≥30% reduction in MRI-PDFF).8
VDFF, Sonic Incytes’ proprietary algorithm that received FDA clearance in 2024, demonstrates a strong correlation (r = 0.85) with MRI-PDFF—the gold standard for liver fat measurement—and achieves an accuracy of 95% of patients with more than 5% MRI-PDFF, defining the presence of hepatic steatosis.9 Velacur ONE combines this technology with a refined user interface, including B-mode imaging—enabling three to four times higher reimbursement than non-imaging elastography—and an AI-based organ overlay feature to aid in liver localization, making it the only point-of-care device that estimates both liver stiffness and attenuation that correlates to MRI-PDFF.10
“The launch of Velacur ONE marks a pivotal milestone for Sonic Incytes as we accelerate our US and global commercial expansion strategy,” says Barry Allen, CEO of Sonic Incytes, in a release. “This next-generation device enhances clinical utility and operational scalability, positioning us to better support the growing demand for accessible, non-invasive liver diagnostics and treatment, particularly in the management of MASLD and MASH at the point-of-care.”
References
- American Liver Foundation. (2025 June). Nonalcoholic fatty liver disease (NAFLD). American Liver Foundation
- Le P, Tatar M, Dasarathy S, et al. Estimated burden of metabolic dysfunction–associated steatotic liver disease in US adults, 2020 to 2050. JAMA Netw Open. 2025;8(1):e2454707.
- Fishman, J., Kim, Y., Charlton, M.R. et al. Estimation of the eligible population for resmetirom among adults in the United States for treatment of non-cirrhotic NASH with moderate-to-advanced liver fibrosis. Adv Ther. 41, 4172–90 (2024).
- Friedman SL. Fat, fibrosis, and the future: navigating the maze of MASLD/MASH. J Clin Invest. 2025 Apr 1;135(7):e186418.
- Fishman J, Alexander T, Kim Y, et al. A clinical decision support tool for metabolic dysfunction-associated steatohepatitis in real-world clinical settings: a mixed-method implementation research study protocol. J Comp Eff Res. 2024 Oct;13(10):e240085.
- Gbadamosi, SO, Evans, KA, Brady, BL, et al. Noninvasive tests and diagnostic pathways to MASH diagnosis in the United States: a retrospective observational study. Journal of Medical Economics. 2025. 28(1), 314–22.
- Noureddin, M, Charlton, MR, Harrison, SA, et al. Expert panel recommendations: Practical clinical applications for initiating and monitoring resmetirom in patients with MASH/NASH and moderate to noncirrhotic advanced fibrosis. Clinical Gastroenterology and Hepatology:22(12), 2367–77.
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025 Jan 1;81(1):312-20.
- Honarvar, M, Lobo, J, Schneider, C, et al. Methods and validation of Velacur determined fat fraction in patients with MASLD. WFUMB Ultrasound Open:2(2), Article 100061.
Photo caption: Velacur ONE
Photo credit: Sonic Incytes