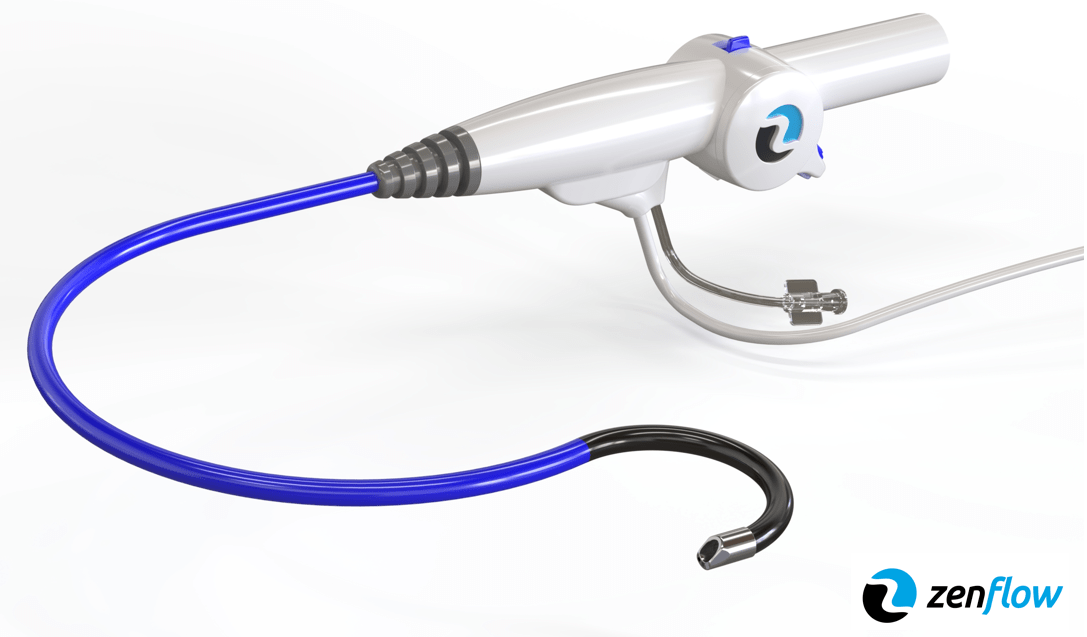
The device features a working channel more than 80% larger than comparable single-use models, aiming to improve visualization during procedures.
Zenflow Inc has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Zenflow Spring Scope & Camera Control Unit, a single-use flexible cystoscope.
According to the company, the device is designed with a 12 French working channel, which is more than 80% larger than current single-use models. The larger channel is intended to enable urologists to perform procedures with improved visualization while maintaining patient comfort.
Flexible cystoscopy is one of the most common office-based procedures performed by urologists for diagnosing and treating lower urinary tract conditions. In the US, approximately 1.2 million cystoscopies are performed annually.
“This new cystoscope technology delivers clear imaging while also allowing us to provide advanced diagnostics and therapeutics in the comfort of our office,” says Bilal Chughtai, MD, chief of urology at Plainview Hospital and investigator of the Zenflow Spring System, in a release. “It’s an exciting and much-anticipated innovation in the field.”
The company states that the Spring Scope is its first product to receive FDA clearance and will also serve as a key component for its forthcoming benign prostatic hyperplasia (BPH) therapy, the Zenflow Spring Implant and Delivery System, which is currently an investigational device.
“With advanced imaging, catheter flexibility, and a large working channel, the Spring Scope is a meaningful innovation in its own right, and a key enabler and differentiator for our forthcoming BPH therapy,” says Shreya Mehta, CEO of Zenflow, in a release. “This progress reflects our commitment to bringing patient-focused innovation to urologists and their patients.”