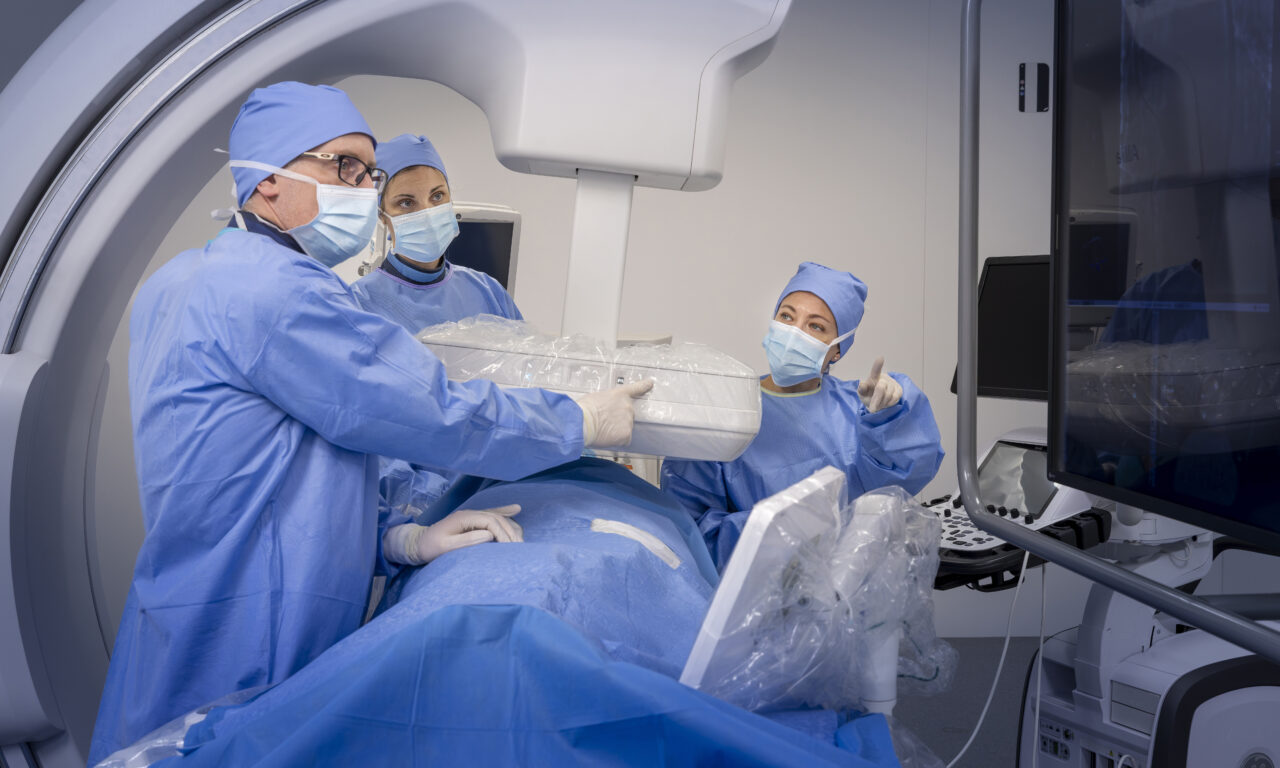
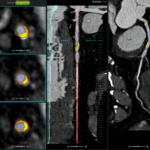
FDA Clears GE HealthCare’s MIM LesionID Pro for Automated Tumor Burden Analysis
The AI-enabled software automates whole-body tumor burden analysis for PSMA PET/CT and SPECT/CT studies to help clinicians assess treatment eligibility and monitor response.